Development of the standard mouse model for human bacterial vaginosis induced by Gardnerella vaginalis
- PMID: 37781285
- PMCID: PMC10536170
- DOI: 10.3389/fvets.2023.1226859
Development of the standard mouse model for human bacterial vaginosis induced by Gardnerella vaginalis
Abstract
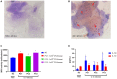
Bacterial vaginosis (BV) is a polymicrobial syndrome characterized by a diminished number of protective bacteria in the vaginal flora. Instead, it is accompanied by a significant increase in facultative and strict anaerobes, including Gardnerella vaginalis (G. vaginalis). BV is one of the most common gynecological problems experienced by reproductive age-women. Because an ideal and standard animal model for human BV induced by G. vaginalis is still underdeveloped, the main objective of this study was to develop a mouse model for human BV induced by G. vaginalis to demonstrate the clinical attributes observed in BV patients. A total of 80 female ICR mice were randomly assigned to 4 groups and intravaginally inoculated with different doses of G. vaginalis: NC (uninfected negative control), PC1 (inoculated with 1 × 105 CFU of G. vaginalis), PC2 (inoculated with 1 × 106 CFU of G. vaginalis) and PC3 (inoculated with 1 × 107 CFU of G. vaginalis). The myeloperoxidase (MPO) activity and serum concentrations of cytokines (IL-1β, IL-10) in mice administered with G. vaginalis were significantly higher than those of the control group. Gross lesion and histopathological analysis of reproductive tract of mice inoculated with G. vaginalis showed inflammation and higher epithelial cell exfoliation compared to the control group. In addition, vaginal swabs from the mice inoculated with G. vaginalis showed the presence of clue cells, which are a characteristic feature of human BV. Altogether, our results suggested that G. vaginalis is sufficient to generate comparable clinical attributes seen in patients with BV.
Keywords: Gardnerella vaginalis; bacterial vaginosis; cytokine; epithelial exfoliation; mouse model.
Copyright © 2023 Kwak, Pandey, Cho, Song, Kim, Doo, Keum, Ryu, Choi, Kang, Kim, Kim and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Clinical features of bacterial vaginosis in a murine model of vaginal infection with Gardnerella vaginalis.PLoS One. 2013;8(3):e59539. doi: 10.1371/journal.pone.0059539. Epub 2013 Mar 19. PLoS One. 2013. PMID: 23527214 Free PMC article.
-
Comparative analysis of virulence factors & biotypes of Gardnerella vaginalis isolated from the genital tract of women with & without bacterial vaginosis.Indian J Med Res. 2019 Jan;149(1):57-61. doi: 10.4103/ijmr.IJMR_1674_16. Indian J Med Res. 2019. PMID: 31115376 Free PMC article.
-
Influence of Biofilm Formation by Gardnerella vaginalis and Other Anaerobes on Bacterial Vaginosis.J Infect Dis. 2015 Dec 15;212(12):1856-61. doi: 10.1093/infdis/jiv338. Epub 2015 Jun 16. J Infect Dis. 2015. PMID: 26080369 Review.
-
Analysis of adherence, biofilm formation and cytotoxicity suggests a greater virulence potential of Gardnerella vaginalis relative to other bacterial-vaginosis-associated anaerobes.Microbiology (Reading). 2010 Feb;156(Pt 2):392-399. doi: 10.1099/mic.0.034280-0. Epub 2009 Nov 12. Microbiology (Reading). 2010. PMID: 19910411 Free PMC article.
-
Gardnerella vaginalis as a Cause of Bacterial Vaginosis: Appraisal of the Evidence From in vivo Models.Front Cell Infect Microbiol. 2020 Apr 24;10:168. doi: 10.3389/fcimb.2020.00168. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32391287 Free PMC article. Review.
Cited by
-
Methanol Extract of Pueraria lobata (Willd.) Root and Its Active Ingredient, Puerarin, Induce Apoptosis in HeLa Cells and Attenuates Bacterial Vaginosis in Gardnerella vaginalis-Infected Mice.Int J Mol Sci. 2025 Feb 5;26(3):1342. doi: 10.3390/ijms26031342. Int J Mol Sci. 2025. PMID: 39941110 Free PMC article.
-
Phenylacetic acid, an anti-vaginitis metabolite produced by the vaginal symbiotic bacterium Chryseobacterium gleum.Sci Rep. 2024 May 28;14(1):12226. doi: 10.1038/s41598-024-62947-7. Sci Rep. 2024. PMID: 38806600 Free PMC article.
-
IL-33 Participates in G. Vaginalis-Induced Bacterial Vaginosis: Involvement of Intravaginal IgA.J Inflamm Res. 2025 Jun 17;18:8005-8013. doi: 10.2147/JIR.S523880. eCollection 2025. J Inflamm Res. 2025. PMID: 40546407 Free PMC article.
References
-
- Paladine HL, Desai UA. Vaginitis: diagnosis and treatment. Am Fam Physician. (2018) 97:321–9. PMID: - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

