A biofunctional review of C-reactive protein (CRP) as a mediator of inflammatory and immune responses: differentiating pentameric and modified CRP isoform effects
- PMID: 37781355
- PMCID: PMC10540681
- DOI: 10.3389/fimmu.2023.1264383
A biofunctional review of C-reactive protein (CRP) as a mediator of inflammatory and immune responses: differentiating pentameric and modified CRP isoform effects
Abstract
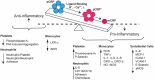
C-reactive protein (CRP) is an acute phase, predominantly hepatically synthesized protein, secreted in response to cytokine signaling at sites of tissue injury or infection with the physiological function of acute pro-inflammatory response. Historically, CRP has been classified as a mediator of the innate immune system, acting as a pattern recognition receptor for phosphocholine-containing ligands. For decades, CRP was envisioned as a single, non-glycosylated, multi-subunit protein arranged non-covalently in cyclic symmetry around a central void. Over the past few years, however, CRP has been shown to exist in at least three distinct isoforms: 1.) a pentamer of five identical globular subunits (pCRP), 2.) a modified monomer (mCRP) resulting from a conformational change when subunits are dissociated from the pentamer, and 3.) a transitional isoform where the pentamer remains intact but is partially changed to express mCRP structural characteristics (referred to as pCRP* or mCRPm). The conversion of pCRP into mCRP can occur spontaneously and is observed under commonly used experimental conditions. In careful consideration of experimental design used in published reports of in vitro pro- and anti-inflammatory CRP bioactivities, we herein provide an interpretation of how distinctive CRP isoforms may have affected reported results. We argue that pro-inflammatory amplification mechanisms are consistent with the biofunction of mCRP, while weak anti-inflammatory mechanisms are consistent with pCRP. The interplay of each CRP isoform with specific immune cells (platelets, neutrophils, monocytes, endothelial cells, natural killer cells) and mechanisms of the innate immune system (complement), as well as differences in mCRP and pCRP ligand recognition and effector functions are discussed. This review will serve as a revised understanding of the structure-function relationship between CRP isoforms as related to inflammation and innate immunity mechanisms.
Keywords: C-reactive protein; CRP isoforms; complement; inflammation; innate immunity; mCRP.
Copyright © 2023 Olson, Hornick, Stefanski, Albanna, Gjoni, Hall, Hart, Rajab and Potempa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

