Pharmacological activation of the amygdala, but not single prolonged footshock-induced acute stress, interferes with cue-induced motivation toward food rewards in rats
- PMID: 37781505
- PMCID: PMC10538645
- DOI: 10.3389/fnbeh.2023.1252868
Pharmacological activation of the amygdala, but not single prolonged footshock-induced acute stress, interferes with cue-induced motivation toward food rewards in rats
Abstract
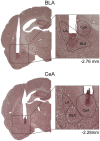
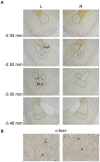
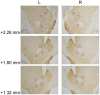
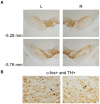
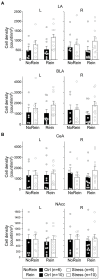
In the face of threats, animals adapt their behaviors to cope with the situation. Under such circumstances, irrelevant behaviors are usually suppressed. In this study, we examined whether food-seeking motivation would decrease under activation of the amygdala, an important nucleus in the regulation of stress response in the central nervous system, or after a physical acute stress session. In Experiment 1, we pharmacologically activated the basolateral nucleus (BLA) or the central nucleus of the amygdala (CeA) before a cue-induced reinstatement test in rats. Our results showed that activation of the BLA or the CeA abolished cue-induced motivation toward food rewards, while locomotor activity and free food intake were not affected. In Experiments 2 and 3, we further assessed anxiety and despair levels, as well as cue-induced reinstatement, after a single prolonged footshock-induced acute stress in rats. Behaviorally, acute stress did not affect anxiety level, despair level, or cue-induced motivation toward food rewards. Physiologically, there was no difference in cellular activities of the amygdala immediately after acute stress. To conclude, our results suggested that pharmacological activation of the amygdala decreased cue-induced motivation toward food reward. However, physiological acute stress did not immediately interfere with the negative emotions, motivation, or amygdala activities of the animals.
Keywords: acute stress; basolateral nucleus of the amygdala; central nucleus of the amygdala; food-seeking; motivated behavior.
Copyright © 2023 Lai and Chang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Activation of the basolateral or the central amygdala dampened the incentive motivation for food reward on high fixed-ratio schedules.Behav Brain Res. 2023 Oct 18;455:114682. doi: 10.1016/j.bbr.2023.114682. Epub 2023 Sep 22. Behav Brain Res. 2023. PMID: 37742807
-
The role of different subregions of the basolateral amygdala in cue-induced reinstatement and extinction of food-seeking behavior.Neuroscience. 2007 Jun 8;146(4):1484-94. doi: 10.1016/j.neuroscience.2007.03.025. Epub 2007 Apr 20. Neuroscience. 2007. PMID: 17449185
-
Single prolonged stress decreases sign-tracking and cue-induced reinstatement of cocaine-seeking.Behav Brain Res. 2019 Feb 1;359:799-806. doi: 10.1016/j.bbr.2018.07.026. Epub 2018 Aug 2. Behav Brain Res. 2019. PMID: 30077578 Free PMC article.
-
Regulation of Alcohol Extinction and Cue-Induced Reinstatement by Specific Projections among Medial Prefrontal Cortex, Nucleus Accumbens, and Basolateral Amygdala.J Neurosci. 2017 Apr 26;37(17):4462-4471. doi: 10.1523/JNEUROSCI.3383-16.2017. Epub 2017 Mar 23. J Neurosci. 2017. PMID: 28336571 Free PMC article.
-
Basolateral amygdala, nicotinic cholinergic receptors, and nicotine: Pharmacological effects and addiction in animal models and humans.Eur J Neurosci. 2019 Aug;50(3):2247-2254. doi: 10.1111/ejn.13970. Epub 2018 Aug 31. Eur J Neurosci. 2019. PMID: 29802666 Review.
References
LinkOut - more resources
Full Text Sources

