Hypoxia-induced exosomes facilitate lung pre-metastatic niche formation in hepatocellular carcinoma through the miR-4508-RFX1-IL17A-p38 MAPK-NF-κB pathway
- PMID: 37781522
- PMCID: PMC10539707
- DOI: 10.7150/ijbs.86767
Hypoxia-induced exosomes facilitate lung pre-metastatic niche formation in hepatocellular carcinoma through the miR-4508-RFX1-IL17A-p38 MAPK-NF-κB pathway
Abstract
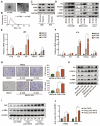
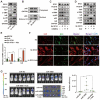
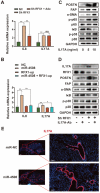
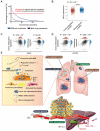
Background: Hypoxia plays an important role in the lung metastasis of hepatocellular carcinoma (HCC). However, the process by which hypoxia promotes the formation of a pre-metastatic niche (PMN) and its underlying mechanism remain unclear. Methods: Exosomes derived from normoxic and hypoxic HCC cells were collected to induce fibroblast activation in vitro and PMN formation in vivo. The micro RNA (miR) profiles of the exosomes were sequenced to identify differentially expressed miRNAs. Gain- and loss-of-function analyses were performed to investigate miR-4508 function. Dual-luciferase, western blotting, and real-time reverse transcription-PCR analyses were used to identify the direct targets of miR-4508 and its downstream signaling pathways. To demonstrate the roles of hypoxic tumor-derived exosomes (H-TDEs) and miR-4508 in the lung metastasis of liver cancer, H22 tumor cells were injected through the tail vein of mice. Blood plasma-derived exosomes from patients with HCC who underwent transarterial chemoembolization (TACE) were applied to determine clinical correlations. Results: We demonstrated that H-TDEs activated lung fibroblasts and facilitated PMN formation, thereby promoting lung metastasis in mice. Screening for upregulated exosomal miRNAs revealed that miR-4508 and its target, regulatory factor X1 (RFX1), were involved in H-TDE-induced lung PMN formation. Moreover, miR-4508 was significantly upregulated in plasma exosomes derived from patients with HCC after TACE. We confirmed that the p38 MAPK-NF-κB signaling pathway is involved in RFX1 knockdown-induced fibroblast activation and PMN formation. In addition, IL17A, a downstream target of RFX1, was identified as a link between RFX1 knockdown and p38 MAPK activation in fibroblasts. Conclusion: Hypoxia enhances the release of TDEs enriched with miR-4508, thereby promoting lung PMN formation by targeting the RFX1-IL17A-p38 MAPK-NF-κB pathway. These findings highlight a novel mechanism underlying hypoxia-induced pulmonary metastasis of HCC.
Keywords: Hypoxia; MicroRNA; Pre-metastatic niche; Tumor-derived exosomes.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Yau T, Chan P, Ng KK. et al. Phase 2 open-label study of single-agent sorafenib in treating advanced hepatocellular carcinoma in a hepatitis B-endemic Asian population: presence of lung metastasis predicts poor response. Cancer. 2009;115(2):428–436. - PubMed
-
- Katyal S, Oliver JH 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216(3):698–703. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

