Rapid, high-titer biosynthesis of melanin using the marine bacterium Vibrio natriegens
- PMID: 37781538
- PMCID: PMC10534004
- DOI: 10.3389/fbioe.2023.1239756
Rapid, high-titer biosynthesis of melanin using the marine bacterium Vibrio natriegens
Abstract
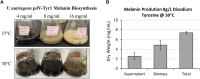
Melanin is one of the most abundant natural biomolecules on Earth. These macromolecular biopolymers display several unique physical and chemical properties and have garnered interest as biomaterials for various commercial and industrial applications. To this end, extensive research has gone into refining methods for the synthesis and extraction of melanin from natural and recombinant sources. In this study, we developed and refined a procedure using a recombinant microbial system for the biosynthesis of melanin using the tyrosinase enzyme Tyr1 and tyrosine as a substrate. Using the emergent microbial chassis organisms Vibrio natriegens, we achieved maximal yields of 7.57 g/L, and one of the highest reported volumetric productivities of 473 mg L-1 h-1 with 100% conversion rates in an optimized, minimally defined medium. Additionally, we identified and investigated the use of a native copper responsive promoter in V. natriegens for stringent regulation of heterologous protein expression as a cost effective alternative to traditional IPTG-based induction. This research represents a promising advancement towards a green, rapid, and economical alternative for the biomanufacture of melanin.
Keywords: Vibrio natriegens; biomanufacturing; copper induction; melanin; melanin biosynthesis; tyrosinase; tyrosine.
Copyright © 2023 Smith, Tschirhart, Compton, Hennessa, VanArsdale and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Cabrera-Valladares N., Martinez A., Pinero S., Lagunas-Munoz V. H., Tinoco R., De Anda R., et al. (2006). Expression of the melA gene from Rhizobium etli CFN42 in Escherichia coli and characterization of the encoded tyrosinase. Enzyme Microb. Technol. 38, 772–779. 10.1016/j.enzmictec.2005.08.004 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

