Short-chain fatty acids ameliorate allergic airway inflammation via sequential induction of PMN-MDSCs and Treg cells
- PMID: 37781663
- PMCID: PMC10509984
- DOI: 10.1016/j.jacig.2023.100163
Short-chain fatty acids ameliorate allergic airway inflammation via sequential induction of PMN-MDSCs and Treg cells
Abstract
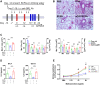
Background: Reinforcement of the immune-regulatory pathway is a feasible strategy for prevention and therapy of allergic asthma. The short-chain fatty acids (SCFAs) acetate, propionate, and butyrate are pleiotropic microbial fermentation products known to induce regulatory T (Treg) cells and exert an immune-regulatory effect. The cellular mechanism underlying SCFA immune regulation in asthma is not fully understood.
Objective: We investigated the role of myeloid-derived suppressor cells (MDSCs) and Treg cells, the immune-regulatory cells of innate and adaptive origin, respectively, in SCFA-elicited protection against allergic airway inflammation.
Methods: BALB/c mice were given SCFA-containing drinking water before being rendered asthmatic in response to ovalbumen. When indicated, mice were given a GR1-depleting antibody to investigate the function of MDSCs in allergic inflammation of the airways. MDSCs were sorted to examine their immunosuppressive function and interaction with T cells.
Results: The mice receiving SCFAs developed less severe asthma that was accompanied by expansion of PMN-MDSCs and Treg cells. Mice depleted of PMN-MDSCs exhibited aggravated asthma, and the protective effect of SCFAs was abrogated after PMN-MDSC depletion. SCFAs were able to directly induce T-cell differentiation toward Treg cells. Additionally, we found that PMN-MDSCs enhanced Treg cell expansion in a cell contact-dependent manner. Whilst membrane-bound TGF-β has been shown to induce Treg cell differentiation, we found that MDSCs upregulated surface expression of TGF-β after coculture with T-cells and that MDSC-induced Treg cell differentiation was partially inhibited by TGF-β blockage.
Conclusions: Although previous studies revealed Treg cells as the effector mechanism of SCFA immune regulation, we found that SCFAs ameliorate allergic airway inflammation by relaying immune regulation, with sequential induction of PMN-MDSCs and Treg cells.
Keywords: Asthma; MDSCs; SCFAs; Treg cells; microbiota; myeloid-derived suppressor cells; short-chain fatty acids.
© 2023 The Author(s).
Figures





References
-
- Duerkop B.A., Vaishnava S., Hooper L.V. Immune responses to the microbiota at the intestinal mucosal surface. Immunity. 2009;31:368–376. - PubMed
-
- Cerf-Bensussan N., Gaboriau-Routhiau V. The immune system and the gut microbiota: friends or foes? Nat Rev Immunol. 2010;10:735–744. - PubMed
-
- Wypych T.P., Wickramasinghe L.C., Marsland B.J. The influence of the microbiome on respiratory health. Nat Immunol. 2019;20:1279–1290. - PubMed
-
- Koh A., De Vadder F., Kovatcheva-Datchary P., Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. - PubMed
LinkOut - more resources
Full Text Sources

