Mechanosensing Piezo channels in gastrointestinal disorders
- PMID: 37781915
- PMCID: PMC10541197
- DOI: 10.1172/JCI171955
Mechanosensing Piezo channels in gastrointestinal disorders
Abstract
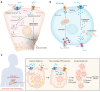
All cells in the body are exposed to physical force in the form of tension, compression, gravity, shear stress, or pressure. Cells convert these mechanical cues into intracellular biochemical signals; this process is an inherent property of all cells and is essential for numerous cellular functions. A cell's ability to respond to force largely depends on the array of mechanical ion channels expressed on the cell surface. Altered mechanosensing impairs conscious senses, such as touch and hearing, and unconscious senses, like blood pressure regulation and gastrointestinal (GI) activity. The GI tract's ability to sense pressure changes and mechanical force is essential for regulating motility, but it also underlies pain originating in the GI tract. Recent identification of the mechanically activated ion channels Piezo1 and Piezo2 in the gut and the effects of abnormal ion channel regulation on cellular function indicate that these channels may play a pathogenic role in disease. Here, we discuss our current understanding of mechanically activated Piezo channels in the pathogenesis of pancreatic and GI diseases, including pancreatitis, diabetes mellitus, irritable bowel syndrome, GI tumors, and inflammatory bowel disease. We also describe how Piezo channels could be important targets for treating GI diseases.
Conflict of interest statement
Figures