Reduction of SPARC protects mice against NLRP3 inflammasome activation and obesity
- PMID: 37781916
- PMCID: PMC10541189
- DOI: 10.1172/JCI169173
Reduction of SPARC protects mice against NLRP3 inflammasome activation and obesity
Abstract
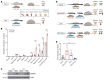
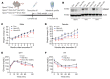
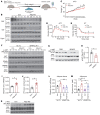
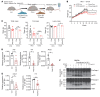
The comprehensive assessment of long-term effects of reducing intake of energy (CALERIE-II; NCT00427193) clinical trial established that caloric restriction (CR) in humans lowers inflammation. The identity and mechanism of endogenous CR-mimetics that can be deployed to control obesity-associated inflammation and diseases are not well understood. Our studies have found that 2 years of 14% sustained CR in humans inhibits the expression of the matricellular protein, secreted protein acidic and rich in cysteine (SPARC), in adipose tissue. In mice, adipose tissue remodeling caused by weight loss through CR and low-protein diet feeding decreased, while high-fat diet-induced (HFD-induced) obesity increased SPARC expression in adipose tissue. Inducible SPARC downregulation in adult mice mimicked CR's effects on lowering adiposity by regulating energy expenditure. Deletion of SPARC in adipocytes was sufficient to protect mice against HFD-induced adiposity, chronic inflammation, and metabolic dysfunction. Mechanistically, SPARC activates the NLRP3 inflammasome at the priming step and downregulation of SPARC lowers macrophage inflammation in adipose tissue, while excess SPARC activated macrophages via JNK signaling. Collectively, reduction of adipocyte-derived SPARC confers CR-like metabolic and antiinflammatory benefits in obesity by serving as an immunometabolic checkpoint of inflammation.
Keywords: Adipose tissue; Inflammation; Innate immunity; Metabolism; Obesity.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

