FTO fuels diabetes-induced vascular endothelial dysfunction associated with inflammation by erasing m6A methylation of TNIP1
- PMID: 37781923
- PMCID: PMC10541204
- DOI: 10.1172/JCI160517
FTO fuels diabetes-induced vascular endothelial dysfunction associated with inflammation by erasing m6A methylation of TNIP1
Abstract
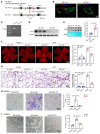
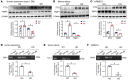
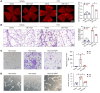
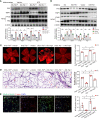
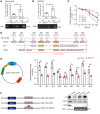
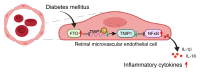
Endothelial dysfunction is a critical and initiating factor of the vascular complications of diabetes. Inflammation plays an important role in endothelial dysfunction regulated by epigenetic modifications. N6-methyladenosine (m6A) is one of the most prevalent epigenetic modifications in eukaryotic cells. In this research, we identified an m6A demethylase, fat mass and obesity-associated protein (FTO), as an essential epitranscriptomic regulator in diabetes-induced vascular endothelial dysfunction. We showed that enhanced FTO reduced the global level of m6A in hyperglycemia. FTO knockdown in endothelial cells (ECs) resulted in less inflammation and compromised ability of migration and tube formation. Compared with EC Ftofl/fl diabetic mice, EC-specific Fto-deficient (EC FtoΔ/Δ) diabetic mice displayed less retinal vascular leakage and acellular capillary formation. Furthermore, methylated RNA immunoprecipitation sequencing (MeRIP-Seq) combined with RNA-Seq indicated that Tnip1 served as a downstream target of FTO. Luciferase activity assays and RNA pull-down demonstrated that FTO repressed TNIP1 mRNA expression by erasing its m6A methylation. In addition, TNIP1 depletion activated NF-κB and other inflammatory factors, which aggravated retinal vascular leakage and acellular capillary formation, while sustained expression of Tnip1 by intravitreal injection of adeno-associated virus alleviated endothelial impairments. These findings suggest that the FTO-TNIP1-NF-κB network provides potential targets to treat diabetic vascular complications.
Keywords: Diabetes; Endothelial cells; Epigenetics; Inflammation; Vascular Biology.
Figures


References
-
- Birkeland KI, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5(9):709–717. doi: 10.1016/S2213-8587(17)30258-9. - DOI - PubMed
-
- Creager MA, et al. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. 2003;108(12):1527–2443. - PubMed

