Characteristics and anatomic location of PD-1+TCF1+ stem-like CD8 T cells in chronic viral infection and cancer
- PMID: 37782797
- PMCID: PMC10576122
- DOI: 10.1073/pnas.2221985120
Characteristics and anatomic location of PD-1+TCF1+ stem-like CD8 T cells in chronic viral infection and cancer
Abstract
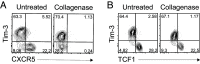
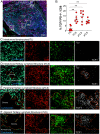
CD8 T cells play an essential role in antitumor immunity and chronic viral infections. Recent findings have delineated the differentiation pathway of CD8 T cells in accordance with the progenitor-progeny relationship of TCF1+ stem-like and Tim-3+TCF1- more differentiated T cells. Here, we investigated the characteristics of stem-like and differentiated CD8 T cells isolated from several murine tumor models and human lung cancer samples in terms of phenotypic and transcriptional features as well as their location compared to virus-specific CD8 T cells in the chronically lymphocytic choriomeningitis virus (LCMV)-infected mice. We found that CD8 tumor-infiltrating lymphocytes (TILs) in both murine and human tumors exhibited overall similar phenotypic and transcriptional characteristics compared to corresponding subsets in the spleen of chronically infected mice. Moreover, stem-like CD8 TILs exclusively responded and produced effector-like progeny CD8 T cells in vivo after antigenic restimulation, confirming their lineage relationship and the proliferative potential of stem-like CD8 TILs. Most importantly, similar to the preferential localization of PD-1+ stem-like CD8 T cells in T cell zones of the spleen during chronic LCMV infection, we found that the PD-1+ stem-like CD8 TILs in lung cancer samples are preferentially located not in the tumor parenchyma but in tertiary lymphoid structures (TLSs). The stem-like CD8 T cells are present in TLSs located within and at the periphery of the tumor, as well as in TLSs closely adjacent to the tumor parenchyma. These findings suggest that TLSs provide a protective niche to support the quiescence and maintenance of stem-like CD8 T cells in the tumor.
Keywords: cancer models; chronic viral infection; human lung cancer; stem-like CD8 T cells; tertiary lymphoid structures.
Conflict of interest statement
The authors declare no competing interest.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

