2-Oxabicyclo[2.2.2]octane as a new bioisostere of the phenyl ring
- PMID: 37783681
- PMCID: PMC10545790
- DOI: 10.1038/s41467-023-41298-3
2-Oxabicyclo[2.2.2]octane as a new bioisostere of the phenyl ring
Abstract
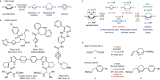
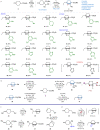
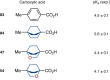
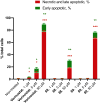
The phenyl ring is a basic structural element in chemistry. Here, we show the design, synthesis, and validation of its new saturated bioisostere with improved physicochemical properties - 2-oxabicyclo[2.2.2]octane. The design of the structure is based on the analysis of the advantages and disadvantages of the previously used bioisosteres: bicyclo[1.1.1]pentane, bicyclo[2.2.2]octane, and cubane. The key synthesis step is the iodocyclization of cyclohexane-containing alkenyl alcohols with molecular iodine in acetonitrile. 2-Oxabicyclo[2.2.2]octane core is incorporated into the structure of Imatinib and Vorinostat (SAHA) drugs instead of the phenyl ring. In Imatinib, such replacement leads to improvement of physicochemical properties: increased water solubility, enhanced metabolic stability, and reduced lipophilicity. In Vorinostat, such replacement results in a new bioactive analog of the drug. This study enhances the repertoire of available saturated bioisosteres of (hetero)aromatic rings for the use in drug discovery projects.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare the following competing interests: V.V.L., Y.P., K.S., O.S., V.B., O.S., I.S., L.B., O.K.-U., Y.H., P.B., K.H., I.B., D.D., P.K.M. are employees of a chemical supplier Enamine. The remaining authors declare no competing interests.
Figures





References
-
- The search was performed at https://go.drugbank.com on 05 July 2023.
LinkOut - more resources
Full Text Sources

