Ex vivo drug response profiling for response and outcome prediction in hematologic malignancies: the prospective non-interventional SMARTrial
- PMID: 37783805
- PMCID: PMC10733146
- DOI: 10.1038/s43018-023-00645-5
Ex vivo drug response profiling for response and outcome prediction in hematologic malignancies: the prospective non-interventional SMARTrial
Abstract
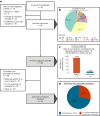
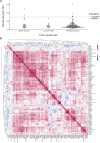
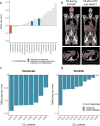
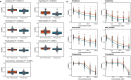
Ex vivo drug response profiling is a powerful tool to study genotype-drug response associations and is being explored as a tool set for precision medicine in cancer. Here we conducted a prospective non-interventional trial to investigate feasibility of ex vivo drug response profiling for treatment guidance in hematologic malignancies (SMARTrial, NCT03488641 ). The primary endpoint to provide drug response profiling reports within 7 d was met in 91% of all study participants (N = 80). Secondary endpoint analysis revealed that ex vivo resistance to chemotherapeutic drugs predicted chemotherapy treatment failure in vivo. We confirmed the predictive value of ex vivo response to chemotherapy in a validation cohort of 95 individuals with acute myeloid leukemia treated with daunorubicin and cytarabine. Ex vivo drug response profiles improved ELN-22 risk stratification in individuals with adverse risk. We conclude that ex vivo drug response profiling is clinically feasible and has the potential to predict chemotherapy response in individuals with hematologic malignancies beyond clinically established genetic markers.
Trial registration: ClinicalTrials.gov NCT03488641 NCT03096821.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures










References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

