Real-world treatment patterns and overall survival among men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) in the US Medicare population
- PMID: 37783836
- PMCID: PMC11096091
- DOI: 10.1038/s41391-023-00725-8
Real-world treatment patterns and overall survival among men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) in the US Medicare population
Abstract
Background: Real-world treatment patterns and survival in metastatic castration-resistant prostate cancer (mCRPC) have not been characterized for the full fee-for-service Medicare population.
Methods: Men newly diagnosed with mCRPC were identified in Medicare fee-for-service claims during 1/1/2014-6/30/2019. Men had evidence of mCRPC and continuous insurance coverage ≥1 year before and ≥6 months after diagnosis unless patients died. Treatment patterns after diagnosis were described. Survival from mCRPC diagnosis and from start of first-line (1 L) therapy was modeled using Kaplan-Meier analysis.
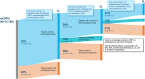
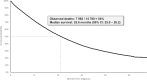
Results: Among 14,780 men with mCRPC, mean age was 76 and median follow-up after mCRPC was 17.0 months. 22% received no life-prolonging therapy after mCRPC, 78% received ≥1 line of therapy (LOT), 42% underwent ≥2 LOTs, and 20% had ≥3 LOTs. Median time from start of 1 L to next LOT or end of follow-up was 13.7 months, 10.9 months from 2 L start, and 8.9 months from 3 L start. The most common 1 L to 2 L treatment sequences among men with ≥2 lines were NHT followed by a different NHT (33%), chemotherapy followed by NHT (14%), and NHT followed by chemotherapy (13%). For those initiating 1 L treatment with NHTs, only 28% received subsequent treatment with a different class of therapy. Median survival was 25.6 months after mCRPC and 23.4 months following treatment initiation.
Conclusions: More than 1 in 5 Medicare patients with mCRPC did not receive any life-prolonging therapy, and less than half received 2 L therapy. NHTs were the most common 1 L and 2 L therapies, with patients treated with NHT as 1 L followed by a different NHT for 2 L as the most common treatment sequence. Median survival from diagnosis for all patients was 25.6 months. These data highlight the dramatic undertreatment that occurs for mCRPC patients, particularly for therapies beyond NHTs as well as the common use of sequential NHTs in real-world data.
© 2023. The Author(s).
Conflict of interest statement
SJF has received consulting fees from Astellas Pharma, AstraZeneca, Bayer, Exact Sciences, Janssen Biotech, Merck, Myovant Sciences, Pfizer, Inc., and Sanofi. MRD and AJE are employees of Medicus Economics, LLC, which received funding from Pfizer to participate in this research. BA and JII are employees and shareholders of Pfizer, Inc.
Figures

References
-
- George DJ, Sartor O, Miller K, Saad F, Tombal B, Kalinovský J, et al. Treatment patterns and outcomes in patients with metastatic castration-resistant prostate cancer in a real-world clinical practice setting in the United States. Clin Genitourin Cancer. 2020;18:284–94. doi: 10.1016/j.clgc.2019.12.019. - DOI - PubMed
-
- Shore ND, Laliberté F, Ionescu-Ittu R, Yang L, Mahendran M, Lejeune D, et al. Real-world treatment patterns and overall survival of patients with metastatic castration-resistant prostate cancer in the US Prior to PARP Inhibitors. Adv Ther. 2021;38:4520–40. doi: 10.1007/s12325-021-01823-6. - DOI - PMC - PubMed
-
- Malangone-Monaco E, Weiyan L, Noxon V, Shan J, Suvina A, Ghate S, et al., editors. Real World Treatment Patterns Among Patients with Metastatic Castration-Resistant Prostate Cancer (mCRPC) in the US. American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium; 2022; San Francisco, CA.
-
- Schultz NM, Flanders SC, Wilson S, Brown BA, Song Y, Yang H, et al. Treatment duration, healthcare resource utilization, and costs among chemotherapy-naïve patients with metastatic castration-resistant prostate cancer treated with enzalutamide or abiraterone acetate: a retrospective claims analysis. Adv Ther. 2018;35:1639–55. doi: 10.1007/s12325-018-0774-1. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

