Generation of Connective Tissue-Free Microvascular Fragment Isolates from Subcutaneous Fat Tissue of Obese Mice
- PMID: 37783934
- PMCID: PMC10645785
- DOI: 10.1007/s13770-023-00571-8
Generation of Connective Tissue-Free Microvascular Fragment Isolates from Subcutaneous Fat Tissue of Obese Mice
Abstract
Background: Microvascular fragment (MVF) isolates are generated by short-term enzymatic digestion of adipose tissue and contain numerous vessel segments for the vascularization of tissue defects. Recent findings indicate that the functionality of these isolates is determined by the quality of the fat source. Therefore, we compared MVF isolates from subcutaneous adipose tissue of obese and lean mice.
Methods: MVF isolates were generated from subcutaneous adipose tissue of donor mice, which received a high fat or control diet for 12 weeks. The isolates were analyzed in vitro and in vivo.
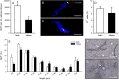
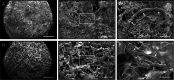
Results: Feeding of mice with a high fat diet induced obesity with adipocyte hypertrophy, resulting in a significantly lower collagen fraction and microvessel density within the subcutaneous fat depots when compared to lean controls. Accordingly, MVF isolates from obese mice also contained a reduced number of MVF per mL adipose tissue. However, these MVF tended to be longer and, in contrast to MVF from lean mice, were not contaminated with collagen fibers. Hence, they could be freely seeded onto collagen-glycosaminoglycan scaffolds, whereas MVF from lean controls were trapped in between large amounts of collagen fibers that clogged the pores of the scaffolds. In line with these results, scaffolds seeded with MVF isolates from obese mice exhibited a significantly improved in vivo vascularization after implantation into full-thickness skin defects.
Conclusion: Subcutaneous adipose tissue from obese mice facilitates the generation of connective tissue-free MVF isolates. Translated to clinical conditions, these findings suggest that particularly obese patients may benefit from MVF-based vascularization strategies.
Keywords: Microvascular fragments; Obesity; Subcutaneous fat tissue; Tissue engineering; Vascularization.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Vascularization of Microvascular Fragment Isolates from Visceral and Subcutaneous Adipose Tissue of Mice.Tissue Eng Regen Med. 2022 Feb;19(1):161-175. doi: 10.1007/s13770-021-00391-8. Epub 2021 Sep 18. Tissue Eng Regen Med. 2022. PMID: 34536211 Free PMC article.
-
Prevascularization of collagen-glycosaminoglycan scaffolds: stromal vascular fraction versus adipose tissue-derived microvascular fragments.J Biol Eng. 2018 Nov 13;12:24. doi: 10.1186/s13036-018-0118-3. eCollection 2018. J Biol Eng. 2018. PMID: 30473729 Free PMC article.
-
Effects of cryopreservation on adipose tissue-derived microvascular fragments.J Tissue Eng Regen Med. 2018 Apr;12(4):1020-1030. doi: 10.1002/term.2591. Epub 2017 Nov 27. J Tissue Eng Regen Med. 2018. PMID: 29047209
-
Microvascular Fragments in Microcirculation Research and Regenerative Medicine.Tissue Eng Part B Rev. 2022 Oct;28(5):1109-1120. doi: 10.1089/ten.TEB.2021.0160. Epub 2022 Jan 10. Tissue Eng Part B Rev. 2022. PMID: 34731017 Review.
-
Adipose tissue and the vascularization of biomaterials: Stem cells, microvascular fragments and nanofat-a review.Cytotherapy. 2020 Aug;22(8):400-411. doi: 10.1016/j.jcyt.2020.03.433. Epub 2020 Jun 2. Cytotherapy. 2020. PMID: 32507607 Review.
Cited by
-
Application and Mechanism of Adipose Tissue-Derived Microvascular Fragments in Tissue Repair and Regeneration.Biomolecules. 2025 Mar 17;15(3):422. doi: 10.3390/biom15030422. Biomolecules. 2025. PMID: 40149958 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
