AZD5582 plus SIV-specific antibodies reduce lymph node viral reservoirs in antiretroviral therapy-suppressed macaques
- PMID: 37783968
- PMCID: PMC10579098
- DOI: 10.1038/s41591-023-02570-7
AZD5582 plus SIV-specific antibodies reduce lymph node viral reservoirs in antiretroviral therapy-suppressed macaques
Abstract
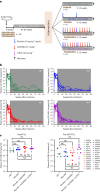
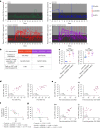
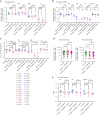
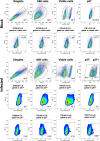
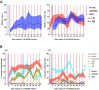
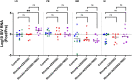
The main barrier to HIV cure is a persistent reservoir of latently infected CD4+ T cells harboring replication-competent provirus that fuels rebound viremia upon antiretroviral therapy (ART) interruption. A leading approach to target this reservoir involves agents that reactivate latent HIV proviruses followed by direct clearance of cells expressing induced viral antigens by immune effector cells and immunotherapeutics. We previously showed that AZD5582, an antagonist of inhibitor of apoptosis proteins and mimetic of the second mitochondrial-derived activator of caspases (IAPi/SMACm), induces systemic reversal of HIV/SIV latency but with no reduction in size of the viral reservoir. In this study, we investigated the effects of AZD5582 in combination with four SIV Env-specific Rhesus monoclonal antibodies (RhmAbs) ± N-803 (an IL-15 superagonist) in SIV-infected, ART-suppressed rhesus macaques. Here we confirm the efficacy of AZD5582 in inducing SIV reactivation, demonstrate enhancement of latency reversal when AZD5582 is used in combination with N-803 and show a reduction in total and replication-competent SIV-DNA in lymph-node-derived CD4+ T cells in macaques treated with AZD5582 + RhmAbs. Further exploration of this therapeutic approach may contribute to the goal of achieving an HIV cure.
© 2023. The Author(s).
Conflict of interest statement
J.T.S. is employed by ImmunityBio. The remaining authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
- R01 OD011095/OD/NIH HHS/United States
- 75N91019D00024/CA/NCI NIH HHS/United States
- UM1 AI126619/AI/NIAID NIH HHS/United States
- R01 AI028433/AI/NIAID NIH HHS/United States
- UM1 AI164567/AI/NIAID NIH HHS/United States
- U54 HL143541/HL/NHLBI NIH HHS/United States
- UM1 AI124436/AI/NIAID NIH HHS/United States
- R37 AI157862/AI/NIAID NIH HHS/United States
- P30 AI050409/AI/NIAID NIH HHS/United States
- R01 AI152703/AI/NIAID NIH HHS/United States
- P51 OD011132/OD/NIH HHS/United States
- P30 AI064518/AI/NIAID NIH HHS/United States
- HHSN261200800001C/RC/CCR NIH HHS/United States
- U42 OD011023/OD/NIH HHS/United States
- P01 AI046518/AI/NIAID NIH HHS/United States
- P01 AI162242/AI/NIAID NIH HHS/United States
- HHSN261200800001E/CA/NCI NIH HHS/United States
- UM1 AI169662/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

