Interplay between ESR1/PIK3CA codon variants, oncogenic pathway alterations and clinical phenotype in patients with metastatic breast cancer (MBC): comprehensive circulating tumor DNA (ctDNA) analysis
- PMID: 37784176
- PMCID: PMC10546685
- DOI: 10.1186/s13058-023-01718-0
Interplay between ESR1/PIK3CA codon variants, oncogenic pathway alterations and clinical phenotype in patients with metastatic breast cancer (MBC): comprehensive circulating tumor DNA (ctDNA) analysis
Abstract
Background: although being central for the biology and druggability of hormone-receptor positive, HER2 negative metastatic breast cancer (MBC), ESR1 and PIK3CA mutations are simplistically dichotomized as mutated or wild type in current clinical practice.
Methods: The study analyzed a multi-institutional cohort comprising 703 patients with luminal-like MBC characterized for circulating tumor DNA through next generation sequencing (NGS). Pathway classification was defined based on previous work (i.e., RTK, RAS, RAF, MEK, NRF2, ER, WNT, MYC, P53, cell cycle, notch, PI3K). Single nucleotide variations (SNVs) were annotated for their oncogenicity through OncoKB. Only pathogenic variants were included in the models. Associations among clinical characteristics, pathway classification, and ESR1/PIK3CA codon variants were explored.
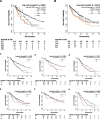
Results: The results showed a differential pattern of associations for ESR1 and PIK3CA codon variants in terms of co-occurring pathway alterations patterns of metastatic dissemination, and prognosis. ESR1 537 was associated with SNVs in the ER and RAF pathways, CNVs in the MYC pathway and bone metastases, while ESR1 538 with SNVs in the cell cycle pathway and liver metastases. PIK3CA 1047 and 542 were associated with CNVs in the PI3K pathway and with bone metastases.
Conclusions: The study demonstrated how ESR1 and PIK3CA codon variants, together with alterations in specific oncogenic pathways, can differentially impact the biology and clinical phenotype of luminal-like MBC. As novel endocrine therapy agents such as selective estrogen receptor degraders (SERDS) and PI3K inhibitors are being developed, these results highlight the pivotal role of ctDNA NGS to describe tumor evolution and optimize clinical decision making.
Keywords: Circulating biomarker; Endocrine therapy; Next generation sequencing.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
L. Gerratana reports consulting or advisory role: AstraZeneca, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Incyte, Novartis, Pfizer, Menarini Stemmline, AbbVie; research funding: Menarini Silicon Biosystems. A. A. Davis reports participating in a scientific advisory board for Pfizer, Inc. F. Puglisi Reports consulting or advisory roles from Roche, MSD, AstraZeneca, Novartis, Lilly, Pfizer, Pierre Fabre, Daiichi Sankyo, Eisai, Amgen, in addition to research funding from Eisai, AstraZeneca, Roche. C.X. Ma holds consultant or advisory roles from Novartis, Seattle Genetics, Agendia, AstraZeneca, Athenex, Bayer HealthCare Pharmaceuticals, Biovica Inc, Eisai, Olaris Inc, Philips Electronics North America, Puma Biotechnology, and SanofiGenzyme, in addition to research funding from Pfizer and Puma Biotechnology. A. Bardia reports consulting or advisory roles with Novartis, Genentech, Pfizer, Spectrum Pharmaceuticals, bioTheranostics, Merck, Radius Health, Immunomedics, Novartis, Genentech/Roche, Radius Health, Innocrin Pharm, Sanofi, Puma Biotechnology, Daiichi Sankyo/Astra Zeneca, Foundation Medicine, Phillips Healthcare. He reports research funding from Genetech (Inst), Novartis (Inst), Pfizer (Inst), Merck (Inst), Sanofi (Inst), Radius Health (Inst), Immunomedics (Inst), Astra Zeneca/Daiichi Sankyo (Inst). M. Cristofanilli reports consulting fees from Novartis, Menarini, Eli Lilly, Sermonix, G1 Therapeutics, Foundation Medicine, Astra Zeneca, Pfizer Inc. Consulting/lecture fees from Foundation Medicine and Pfizer Inc. Travel support from Foundation Medicine. Participation on a Date Safety Monitoring Board or Advisory Board for Merck and Astra Zenca. Research funding from Pfizer Inc, Menarini, Eli Lilly, and G1 Therapuetics. The remaining authors report no conflicts of interest.
Figures



References
-
- Criscitiello C, Marra A, Curigliano G. PIK3CA mutation assessment in HR+/HER2− metastatic breast cancer: overview for oncology clinical practice. J Mol Pathol. 2021;2:42–54. doi: 10.3390/jmp2010005. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

