Higher angiotensin-converting enzyme 2 (ACE2) levels in the brain of individuals with Alzheimer's disease
- PMID: 37784209
- PMCID: PMC10544218
- DOI: 10.1186/s40478-023-01647-1
Higher angiotensin-converting enzyme 2 (ACE2) levels in the brain of individuals with Alzheimer's disease
Erratum in
-
Correction to: Higher angiotensin‑converting enzyme 2 (ACE2) levels in the brain of individuals with Alzheimer's disease.Acta Neuropathol Commun. 2023 Nov 1;11(1):173. doi: 10.1186/s40478-023-01678-8. Acta Neuropathol Commun. 2023. PMID: 37915100 Free PMC article. No abstract available.
Abstract
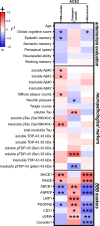
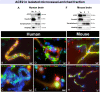
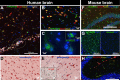
Cognitive decline due to Alzheimer's disease (AD) is frequent in the geriatric population, which has been disproportionately affected by the COVID-19 pandemic. In this study, we investigated the levels of angiotensin-converting enzyme 2 (ACE2), a regulator of the renin-angiotensin system and the main entry receptor of SARS-CoV-2 in host cells, in postmortem parietal cortex samples from two independent AD cohorts, totalling 142 persons. Higher concentrations of ACE2 protein (p < 0.01) and mRNA (p < 0.01) were found in individuals with a neuropathological diagnosis of AD compared to age-matched healthy control subjects. Brain levels of soluble ACE2 were inversely associated with cognitive scores (p = 0.02) and markers of pericytes (PDGFRβ, p = 0.02 and ANPEP, p = 0.007), but positively correlated with concentrations of soluble amyloid-β peptides (Aβ) (p = 0.01) and insoluble phospho-tau (S396/404, p = 0.002). However, no significant differences in ACE2 were observed in the 3xTg-AD mouse model of tau and Aβ neuropathology. Results from immunofluorescence and Western blots showed that ACE2 protein is predominantly localized in microvessels in the mouse brain whereas it is more frequently found in neurons in the human brain. The present data suggest that higher levels of soluble ACE2 in the human brain may contribute to AD, but their role in CNS infection by SARS-CoV-2 remains unclear.
Keywords: ACE2; Alzheimer’s disease; Blood–brain barrier; Cognitive dysfunction; Neuropathology.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors report no competing interests.
Figures



Update of
-
Higher Angiotensin I Converting Enzyme 2 (ACE2) levels in the brain of individuals with Alzheimer's disease.bioRxiv [Preprint]. 2023 Jan 18:2023.01.17.524254. doi: 10.1101/2023.01.17.524254. bioRxiv. 2023. Update in: Acta Neuropathol Commun. 2023 Oct 2;11(1):159. doi: 10.1186/s40478-023-01647-1. PMID: 36711734 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

