Diet and gut microbial associations in irritable bowel syndrome according to disease subtype
- PMID: 37786251
- PMCID: PMC10549191
- DOI: 10.1080/19490976.2023.2262130
Diet and gut microbial associations in irritable bowel syndrome according to disease subtype
Abstract
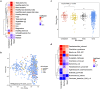
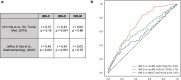
The role of diet and the gut microbiome in the etiopathogenesis of irritable bowel syndrome (IBS) is not fully understood. Therefore, we investigated the interplay between dietary risk factors and gut microbiota in IBS subtypes using a food frequency questionnaire and stool metagenome data from 969 participants aged 18-65 years in the ZOE PREDICT 1 study, an intervention study designed to predict postprandial metabolic responses. We identified individuals with IBS subtype according to the Rome III criteria based on predominant bowel habits during symptom onset: diarrhea (i.e. looser), constipation (i.e. harder), and mixed. Participants with IBS-D (n = 59) consumed more healthy plant-based foods (e.g. whole grains, leafy vegetables) and fiber, while those with IBS-C (n = 49) tended to consume more unhealthy plant-based foods (e.g. refined grains, fruit juice) than participants without IBS (n = 797). Microbial diversity was nominally lower in patients with IBS-D than in participants without IBS or with IBS-C. Using multivariable-adjusted linear regression, we identified specific microbiota variations in IBS subtypes, including slight increases in pro-inflammatory taxa in IBS-C (e.g. Escherichia coli) and loss of strict anaerobes in IBS-D (e.g. Faecalibacterium prausnitzii). Our analysis also revealed intriguing evidence of interactions between diet and Faecalibacterium prausnitzii. The positive associations between fiber and iron intake and IBS-diarrhea were stronger among individuals with a higher relative abundance of Faecalibacterium prausnitzii, potentially driven by carbohydrate metabolic pathways, including the superpathway of β-D-glucuronide and D-glucuronate degradation. In conclusion, our findings suggest subtype-specific variations in dietary habits, gut microbial composition and function, and diet-microbiota interactions in IBS, providing insights into potential microbiome-informed dietary interventions.
Keywords: Fiber; diarrhea; functional bowel disorder; glycan metabolism; microbial enzymes.
Conflict of interest statement
ZOE PREDICT 1 was supported by Zoe Ltd., for which Dr. Chan and Dr. Drew served as investigators. Dr. Chan served as a consultant for Pfizer Inc. and Boehringer Ingelheim for unrelated studies and a grant support from Pfizer Inc. and Freenome for unrelated studies. Dr. Huttenhower and Dr. Segata are on the Scientific Advisory Board of Zoe Ltd. Dr. Berry and Dr. Spector have equity in Zoe Ltd. Mr. Wolf is an employee of Zoe Ltd. Dr. Staller has received research support from Ironwood and Urovant and has served as a consultant for Anji, Ardelyx, Arena, Gelesis, GI Supply, Restalsis, and Sanofi. No other disclosures have been reported.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
