Atherosclerosis Progression in the APPLE Trial Can Be Predicted in Young People With Juvenile-Onset Systemic Lupus Erythematosus Using a Novel Lipid Metabolomic Signature
- PMID: 37786302
- PMCID: PMC10922368
- DOI: 10.1002/art.42722
Atherosclerosis Progression in the APPLE Trial Can Be Predicted in Young People With Juvenile-Onset Systemic Lupus Erythematosus Using a Novel Lipid Metabolomic Signature
Abstract
Objective: Patients with juvenile-onset systemic lupus erythematosus (JSLE) have increased atherosclerosis risk. This study investigated novel atherosclerosis progression biomarkers in the Atherosclerosis Prevention in Pediatric Lupus Erythematosus (APPLE) trial, the largest investigator-led randomized control trial of atorvastatin versus placebo for atherosclerosis progression in JSLE, using carotid intima-media thickness (CIMT) as the primary outcome.
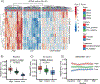
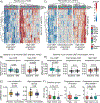
Methods: Unsupervised clustering of baseline CIMT and CIMT progression over 36 months was used to stratify patients with JSLE. Disease characteristics, cardiovascular risk scores, and baseline serum metabolome were investigated in CIMT-stratified patients. Machine learning techniques were used to identify and validate a serum metabolomic signature of CIMT progression.
Results: Baseline CIMT stratified patients with JSLE (N = 151) into three groups with distinct high, intermediate, and low CIMT trajectories irrespective of treatment allocation, despite most patients having low cardiovascular disease risk based on recommended assessment criteria. In the placebo group (n = 60), patients with high versus low CIMT progression had higher total (P = 0.001) and low-density lipoprotein (LDL) (P = 0.002) cholesterol levels, although within the reference range. Furthermore, a robust baseline metabolomic signature predictive of high CIMT progression was identified in the placebo arm (area under the curve, 80.7%). Patients treated with atorvastatin (n = 61) had reduced LDL cholesterol levels after 36 months, as expected; however, despite this, 36% still had high atherosclerosis progression, which was not predicted by metabolomic biomarkers, suggesting nonlipid drivers of atherosclerosis in JSLE with management implications for this subset of patients.
Conclusion: Significant baseline heterogeneity and distinct subclinical atherosclerosis progression trajectories exist in JSLE. Metabolomic signatures can predict atherosclerosis progression in some patients with JSLE with relevance for clinical trial stratification.
© 2023 The Authors. Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Conflict of interest statement
Competing interests
The authors declared no relevant conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

