Reductive Coupling of a Diazoalkane Derivative Promoted by a Potassium Aluminyl and Elimination of Dinitrogen to Generate a Reactive Aluminium Ketimide
- PMID: 37786384
- PMCID: PMC10946750
- DOI: 10.1002/chem.202302903
Reductive Coupling of a Diazoalkane Derivative Promoted by a Potassium Aluminyl and Elimination of Dinitrogen to Generate a Reactive Aluminium Ketimide
Abstract
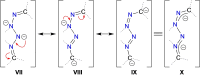
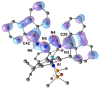
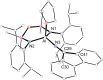
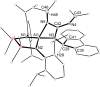
The reaction of 9-diazo-9H-fluorene (fluN2 ) with the potassium aluminyl K[Al(NON)] ([NON]2- =[O(SiMe2 NDipp)2 ]2- , Dipp=2,6-iPr2 C6 H3 ) affords K[Al(NON)(κN1 ,N3 -{(fluN2 )2 })] (1). Structural analysis shows a near planar 1,4-di(9H-fluoren-9-ylidene)tetraazadiide ligand that chelates to the aluminium. The thermally induced elimination of dinitrogen from 1 affords the neutral aluminium ketimide complex, Al(NON)(N=flu)(THF) (2) and the 1,2-di(9H-fluoren-9-yl)diazene dianion as the potassium salt, [K2 (THF)3 ][fluN=Nflu] (3). The reaction of 2 with N,N'-diisopropylcarbodiimide (iPrN=C=NiPr) affords the aluminium guanidinate complex, Al(NON){N(iPr)C(N=CMe2 )N(CHflu)} (4), showing a rare example of reactivity at a metal ketimide ligand. Density functional theory (DFT) calculations have been used to examine the bonding in the newly formed [(fluN2 )2 ]2- ligand in 1 and the ketimide bonding in 2. The mechanism leading to the formation of 4 has also been studied using this technique.
Keywords: aluminyl; diazomethane; guanidinate; ketimide; reductive coupling.
© 2023 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Potassium Aluminyl Promoted Carbonylation of Ethene.Angew Chem Int Ed Engl. 2022 Apr 11;61(16):e202117396. doi: 10.1002/anie.202117396. Epub 2022 Feb 23. Angew Chem Int Ed Engl. 2022. PMID: 35166007 Free PMC article.
-
Carbon-Carbon Bond Forming Reactions Promoted by Aluminyl and Alumoxane Anions: Introducing the Ethenetetraolate Ligand.Angew Chem Int Ed Engl. 2020 Jul 27;59(31):12806-12810. doi: 10.1002/anie.202005301. Epub 2020 Jun 2. Angew Chem Int Ed Engl. 2020. PMID: 32378311
-
Dihydrogen Activation by Lithium- and Sodium-Aluminyls.Angew Chem Int Ed Engl. 2021 Oct 4;60(41):22289-22292. doi: 10.1002/anie.202108934. Epub 2021 Aug 31. Angew Chem Int Ed Engl. 2021. PMID: 34402149
-
Reduction vs. Addition: The Reaction of an Aluminyl Anion with 1,3,5,7-Cyclooctatetraene.Angew Chem Int Ed Engl. 2019 Jan 28;58(5):1489-1493. doi: 10.1002/anie.201811675. Epub 2018 Dec 28. Angew Chem Int Ed Engl. 2019. PMID: 30548141
-
The emerging chemistry of the aluminyl anion.Chem Commun (Camb). 2023 Jan 12;59(5):503-519. doi: 10.1039/d2cc05963k. Chem Commun (Camb). 2023. PMID: 36541674 Review.
Cited by
-
Alumanyl Reduction, Reductive Coupling and C-H Isomerization of Organic Nitriles.Organometallics. 2024 Aug 16;43(17):1938-1945. doi: 10.1021/acs.organomet.4c00289. eCollection 2024 Sep 9. Organometallics. 2024. PMID: 39268183 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

