Early Metformin in Gestational Diabetes: A Randomized Clinical Trial
- PMID: 37786390
- PMCID: PMC10548359
- DOI: 10.1001/jama.2023.19869
Early Metformin in Gestational Diabetes: A Randomized Clinical Trial
Abstract
Importance: Gestational diabetes is a common complication of pregnancy and the optimal management is uncertain.
Objective: To test whether early initiation of metformin reduces insulin initiation or improves fasting hyperglycemia at gestation weeks 32 or 38.
Design, setting, and participants: Double-blind, placebo-controlled trial conducted in 2 centers in Ireland (one tertiary hospital and one smaller regional hospital). Participants were enrolled from June 2017 through September 2022 and followed up until 12 weeks' postpartum. Participants comprised 510 individuals (535 pregnancies) diagnosed with gestational diabetes based on World Health Organization 2013 criteria.
Interventions: Randomized 1:1 to either placebo or metformin (maximum dose, 2500 mg) in addition to usual care.
Main outcomes and measures: The primary outcome was a composite of insulin initiation or a fasting glucose level of 5.1 mmol/L or greater at gestation weeks 32 or 38.
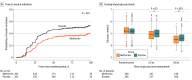
Results: Among 510 participants (mean age, 34.3 years), 535 pregnancies were randomized. The primary composite outcome was not significantly different between groups and occurred in 150 pregnancies (56.8%) in the metformin group and 167 pregnancies (63.7%) in the placebo group (between-group difference, -6.9% [95% CI, -15.1% to 1.4%]; relative risk, 0.89 [95% CI, 0.78-1.02]; P = .13). Of 6 prespecified secondary maternal outcomes, 3 favored the metformin group, including time to insulin initiation, self-reported capillary glycemic control, and gestational weight gain. Secondary neonatal outcomes differed by group, with smaller neonates (lower mean birth weights, a lower proportion weighing >4 kg, a lower proportion in the >90% percentile, and smaller crown-heel length) in the metformin group without differences in neonatal intensive care needs, respiratory distress requiring respiratory support, jaundice requiring phototherapy, major congenital anomalies, neonatal hypoglycemia, or proportion with 5-minute Apgar scores less than 7.
Conclusion and relevance: Early treatment with metformin was not superior to placebo for the composite primary outcome. Prespecified secondary outcome data support further investigation of metformin in larger clinical trials.
Trial registration: ClinicalTrials.gov Identifier: NCT02980276; EudraCT: 2016-001644-19.
Conflict of interest statement
Figures

Comment in
-
Metformin for Diabetes in Pregnancy: Are We Closer to Defining Its Role?JAMA. 2023 Dec 12;330(22):2167-2169. doi: 10.1001/jama.2023.18589. JAMA. 2023. PMID: 38085322 No abstract available.
-
Early Metformin Treatment for Gestational Diabetes.JAMA. 2024 Feb 20;331(7):618. doi: 10.1001/jama.2023.27793. JAMA. 2024. PMID: 38497700 No abstract available.
-
Early Metformin Treatment for Gestational Diabetes.JAMA. 2024 Feb 20;331(7):617-618. doi: 10.1001/jama.2023.27790. JAMA. 2024. PMID: 38497701 No abstract available.
References
-
- World Health Organization . Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. Published January 1, 2013. Accessed July 1, 2023. https://www.who.int/publications/i/item/WHO-NMH-MND-13.2 - PubMed
-
- Yuen L, Saeedi P, Riaz M, et al. Projections of the prevalence of hyperglycaemia in pregnancy in 2019 and beyond: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. Published online September 10, 2019. doi: 10.1016/j.diabres.2019.107841 - DOI - PubMed
-
- O’Sullivan EP, Avalos G, O’Reilly M, Dennedy MC, Gaffney G, Dunne F; Atlantic DIP Collaborators . Atlantic Diabetes in Pregnancy (DIP): the prevalence and outcomes of gestational diabetes mellitus using new diagnostic criteria. Diabetologia. 2011;54(7):1670-1675. doi: 10.1007/s00125-011-2150-4 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

