Revalidation of the ATTRACTION-4 study in a real-world setting: a multicenter, retrospective propensity score matching study in China
- PMID: 37786611
- PMCID: PMC10541969
- DOI: 10.3389/fimmu.2023.1264929
Revalidation of the ATTRACTION-4 study in a real-world setting: a multicenter, retrospective propensity score matching study in China
Abstract
Background: Immune-checkpoint inhibitors (ICIs) combined with chemotherapy have been successfully used in clinical trials to treat advanced gastric cancer. However, the efficacy and safety of first-line immunotherapy combined with chemotherapy in Chinese patients are unknown.
Methods: This multicenter retrospective study included patients with human epidermal growth factor receptor-2 (HER-2) negative advanced gastric cancer treated with first-line chemotherapy or chemotherapy with an ICI between January 2019 and December 2022. Propensity score matching was used to compare progression-free survival (PFS), overall survival, objective response rates, and adverse reactions between cohorts.
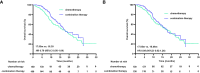
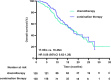
Results: After propensity score matching, 138 patients, who had balanced baseline characteristics, were included in the chemotherapy and combination treatment groups. The median follow-up duration was 16.90 months, and the median PFS was 8.53 months (95% confidence interval [CI] 7.77-9.28) in the combination treatment group and 5.97 months (95% CI 4.56-7.37) in the chemotherapy group. The median survival duration was 17.05 months (95% CI 14.18-19.92) in the combination treatment group and 16.46 months (95% CI 12.99-19.93) in the chemotherapy group. The PFS subgroup analysis revealed that age ≥65 years, women, Eastern Cooperative Oncology Group performance status of 1, non-signet ring cell carcinoma, esophagogastric junction, liver metastasis, peritoneal metastasis, no massive ascites, only one metastatic organ, and combined platinum-based chemotherapy correlated with treatment benefit. The incidences of adverse events above grade 3 were comparable between groups.
Conclusions: Our study confirmed the ATTRACTION-4 trial results. Compared with chemotherapy, first-line ICIs combined with chemotherapy prolonged PFS but did not improve overall survival in patients with HER-2-negative advanced gastric cancer.
Keywords: advanced gastric cancer; immune checkpoint inhibitors; overall survival; progression free survival; propensity score matching.
Copyright © 2023 Dai, Liu, Gong, He, Wang, Yang, Qiu, Zhang, Yuan, Cheng and Qiu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Immunotherapy plus chemotherapy versus chemotherapy alone in the first-line treatment for advanced gastric cancer/gastroesophageal junction cancer: a real-world retrospective study.Front Immunol. 2024 Nov 7;15:1463017. doi: 10.3389/fimmu.2024.1463017. eCollection 2024. Front Immunol. 2024. PMID: 39575245 Free PMC article.
-
Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial.Lancet Oncol. 2022 Feb;23(2):234-247. doi: 10.1016/S1470-2045(21)00692-6. Epub 2022 Jan 11. Lancet Oncol. 2022. PMID: 35030335 Clinical Trial.
-
Pertuzumab in combination with trastuzumab and chemotherapy for Chinese patients with HER2-positive metastatic gastric or gastroesophageal junction cancer: a subpopulation analysis of the JACOB trial.Cancer Commun (Lond). 2019 Jun 24;39(1):38. doi: 10.1186/s40880-019-0384-6. Cancer Commun (Lond). 2019. PMID: 31234927 Free PMC article. Clinical Trial.
-
Comparison of a combination of chemotherapy and immune checkpoint inhibitors and immune checkpoint inhibitors alone for the treatment of advanced and metastatic non-small cell lung cancer.Thorac Cancer. 2019 May;10(5):1158-1166. doi: 10.1111/1759-7714.13057. Epub 2019 Apr 5. Thorac Cancer. 2019. PMID: 30950239 Free PMC article.
-
First-line immune-checkpoint inhibitor combination therapy for chemotherapy-eligible patients with metastatic urothelial carcinoma: A systematic review and meta-analysis.Eur J Cancer. 2021 Jul;151:35-48. doi: 10.1016/j.ejca.2021.03.049. Epub 2021 May 4. Eur J Cancer. 2021. PMID: 33962359
Cited by
-
Exploration of Constitutional Bias and Associated Factors in Patients with Recurrent and Metastatic Gastric Cancer, and the Therapeutic Effect of Yiqi Wenyang Jiedu Prescription: A one-arm study.Integr Cancer Ther. 2025 Jan-Dec;24:15347354251331100. doi: 10.1177/15347354251331100. Epub 2025 Apr 18. Integr Cancer Ther. 2025. PMID: 40248901 Free PMC article. Clinical Trial.
-
Efficacy and safety of sintilimab combined with trastuzumab and chemotherapy in HER2-positive advanced gastric or gastroesophageal junction cancer.Front Immunol. 2025 Feb 14;16:1545304. doi: 10.3389/fimmu.2025.1545304. eCollection 2025. Front Immunol. 2025. PMID: 40028325 Free PMC article.
-
Treatment patterns and outcomes in advanced or metastatic gastric/gastroesophageal junction adenocarcinoma in China.Future Oncol. 2025 Apr;21(10):1179-1188. doi: 10.1080/14796694.2025.2476930. Epub 2025 Mar 17. Future Oncol. 2025. PMID: 40091795 Free PMC article.
-
Immunosuppressive tumor microenvironment in gastric signet-ring cell carcinoma.World J Clin Oncol. 2024 Sep 24;15(9):1126-1131. doi: 10.5306/wjco.v15.i9.1126. World J Clin Oncol. 2024. PMID: 39351457 Free PMC article.
-
Two autopsied gastric cancer cases of rare drug-induced pneumonia associated with nivolumab plus S-1 and oxaliplatin: a case report.J Gastrointest Oncol. 2024 Feb 29;15(1):491-499. doi: 10.21037/jgo-23-511. Epub 2024 Feb 1. J Gastrointest Oncol. 2024. PMID: 38482223 Free PMC article.
References
-
- Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, et al. . Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2–negative, untreated, unresectable advanced or recurrent gastric or gastro–oesophageal junction cancer (ATTRACTION–4): a randomised, multicentre, double–blind, placebo–controlled, phase 3 trial. Lancet Oncol (2022) 23(2):234–47. doi: 10.1016/S1470-2045(21)00692-6 - DOI - PubMed
-
- Xu J–M, Jiang H, Pan Y, Gu K, Cang S, Han L, et al. . Abstract CT078: First–line treatment with sintilimab (sin) vs placebo in combination with chemotherapy (chemo) in patients (pts) with unresectable gastric or gastroesophageal junction (G/GEJ) cancer: Final overall survival (OS) results from the randomized, phase III ORIENT–16 trial. Cancer Res (2023) 83:CT078–CT. doi: 10.1158/1538-7445 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

