This is a preprint.
Structure of RADX and mechanism for regulation of RAD51 nucleofilaments
- PMID: 37786681
- PMCID: PMC10541619
- DOI: 10.1101/2023.09.19.558089
Structure of RADX and mechanism for regulation of RAD51 nucleofilaments
Update in
-
Structure of RADX and mechanism for regulation of RAD51 nucleofilaments.Proc Natl Acad Sci U S A. 2024 Mar 19;121(12):e2316491121. doi: 10.1073/pnas.2316491121. Epub 2024 Mar 11. Proc Natl Acad Sci U S A. 2024. PMID: 38466836 Free PMC article.
Abstract
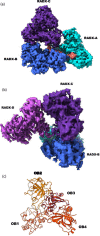
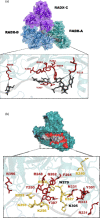
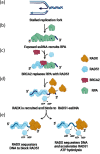
Replication fork reversal is a fundamental process required for resolution of encounters with DNA damage. A key step in the stabilization and eventual resolution of reversed forks is formation of RAD51 nucleoprotein filaments on exposed ssDNA. To avoid genome instability, RAD51 filaments are tightly controlled by a variety of positive and negative regulators. RADX is a recently discovered negative regulator that binds tightly to ssDNA, directly interacts with RAD51, and regulates replication fork reversal and stabilization in a context-dependent manner. Here we present a structure-based investigation of RADX's mechanism of action. Mass photometry experiments showed that RADX forms multiple oligomeric states in a concentration dependent manner, with a predominance of trimers in the presence of ssDNA. The structure of RADX, which has no structurally characterized orthologs, was determined ab initio by cryo-electron microscopy (EM) from maps in the 2-3 Å range. The structure reveals the molecular basis for RADX oligomerization and binding of ssDNA binding. The binding of RADX to RAD51 filaments was imaged by negative stain EM, which showed a RADX oligomer at the end of filaments. Based on these results, we propose a model in which RADX functions by capping and restricting the growing end of RAD51 filaments.
Keywords: Biological Sciences; Biophysics and Computational Biology; DNA replication; RAD51; cryo-EM; replication fork.
Conflict of interest statement
Competing interest statement: The authors declare that they have no conflicts of interest.
Figures




References
-
- Berti M., Cortez D., and Lopes M. (2020) The plasticity of DNA replication forks in response to clinically relevant genotoxic stress. Nat. Rev. Mol. Cell Biol. 21, 633–651. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
