Ubiquitination detection techniques
- PMID: 37787047
- PMCID: PMC10625345
- DOI: 10.1177/15353702231191186
Ubiquitination detection techniques
Abstract
Ubiquitination is an intricately regulated post-translational modification that involves the covalent attachment of ubiquitin to a substrate protein. The complex dynamic nature of the ubiquitination process regulates diverse cellular functions including targeting proteins for degradation, cell cycle, deoxyribonucleic acid (DNA) damage repair, and numerous cell signaling pathways. Ubiquitination also serves as a crucial mechanism in protein quality control. Dysregulation in ubiquitination could result in lethal disease conditions such as cancers and neurodegenerative diseases. Therefore, the ubiquitination cascade has become an attractive target for therapeutic interventions. Enormous efforts have been made to detect ubiquitination involving different detection techniques to better grasp the underlying molecular mechanisms of ubiquitination. This review discusses a wide range of techniques stretching from the simplest assays to real-time assays. This includes western blotting/immunoblotting, fluorescence assays, chemiluminescence assays, spectrophotometric assays, and nanopore sensing assays. This review compares these applications, and the inherent advantages and limitations.
Keywords: Ubiquitination; chemiluminescence; detection techniques; fluorescence; spectrophotometric.
Conflict of interest statement
Declaration Of Conflicting InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
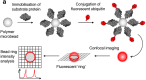


References
-
- Sun L, Chen ZJ. The novel functions of ubiquitination in signaling. Curr Opin Cell Biol 2004;16:119–26 - PubMed
-
- Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat Med 2014;20:1242–53 - PubMed
-
- Finley D, Ciechanover A, Varshavsky A. Ubiquitin as a central cellular regulator. Cell 2004;116:S29–32 - PubMed
-
- Haas AL, Rose IA. The mechanism of ubiquitin activating enzyme. A kinetic and equilibrium analysis. J Biol Chem 1982;257:10329–37 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

