Evidence for Tuberculosis in Individuals With Xpert Ultra "Trace" Sputum During Screening of High-Burden Communities
- PMID: 37787077
- PMCID: PMC10954329
- DOI: 10.1093/cid/ciad595
Evidence for Tuberculosis in Individuals With Xpert Ultra "Trace" Sputum During Screening of High-Burden Communities
Abstract
Background: "Trace" results on Xpert MTB/RIF Ultra ("Ultra"; Cepheid) -a molecular diagnostic test for tuberculosis (TB)-are often interpreted as an indication for TB treatment, but may also represent detection of nonviable bacilli or analytical error. In community-screening settings where individual TB risk is low, there is limited guidance on how to interpret Ultra-trace results.
Methods: We conducted systematic Ultra TB screening of adults and adolescents (≥15 years) in Kampala, Uganda, through door-to-door and event-based sputum collection. We enrolled individuals with trace-positive sputum for detailed clinical, radiographic, and microbiological (including 2 sputum cultures, repeat Ultra, and for people with HIV, urine lipoarabinomannan) evaluation, and compared those findings with similar evaluations in controls with Ultra-negative and Ultra-positive (non-trace) sputum.
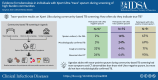
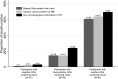
Results: Of 21 957 people screened with Ultra, 211 (1.0%) tested positive, including 96 (46% of positives) with trace results. Of 92 people enrolled with trace-positive sputum; 12% (11/92) were HIV-positive and 14% (13/92) had prior TB. The prevalence of TB among participants with trace-positive sputum results was 14% (13/92) by culture, 24% (22/92) using broader microbiological criteria, and 26% (24/92) after accounting for clinical diagnosis. The prevalence of cough and of abnormal chest computed tomography (CT) findings were 32% and 26%, respectively, if Ultra-negative; 34% and 54% if trace-positive/non-microbiologically confirmed; 72% and 95% if trace-positive/microbiologically confirmed; and 71% and 93% if Ultra-positive (more than trace).
Conclusions: Most individuals with trace-positive sputum in Ugandan communities did not have microbiologically confirmed TB but had more symptoms and chest CT abnormalities than people with Ultra-negative sputum.
Keywords: Xpert MTB/RIF Ultra; Xpert Ultra; community screening; trace; tuberculosis.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Figures
References
-
- WHO Guidelines Review Committee . WHO consolidated guidelines on tuberculosis: module 3: diagnosis—rapid diagnostics for tuberculosis detection. Geneva, Switzerland: World Health Organization, 2021. - PubMed
-
- Opota O, Mazza-Stalder J, Greub G, Jaton K. The rapid molecular test Xpert MTB/RIF Ultra: towards improved tuberculosis diagnosis and rifampicin resistance detection. Clin Microbiol Infect 2019; 25:1370–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

