DegU-mediated suppression of carbohydrate uptake in Listeria monocytogenes increases adaptation to oxidative stress
- PMID: 37787570
- PMCID: PMC10617591
- DOI: 10.1128/aem.01017-23
DegU-mediated suppression of carbohydrate uptake in Listeria monocytogenes increases adaptation to oxidative stress
Abstract
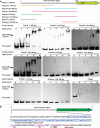
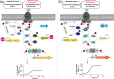
The foodborne bacterial pathogen Listeria monocytogenes exhibits remarkable survival capabilities under challenging conditions, severely threatening food safety and human health. The orphan regulator DegU is a pleiotropic regulator required for bacterial environmental adaptation. However, the specific mechanism of how DegU participates in oxidative stress tolerance remains unknown in L. monocytogenes. In this study, we demonstrate that DegU suppresses carbohydrate uptake under stress conditions by altering global transcriptional profiles, particularly by modulating the transcription of the phosphoenolpyruvate-carbohydrate phosphotransferase system (PTS)-related genes, such as ptsH, ptsI, and hprK. Specifically, in the absence of degU, the transcripts of ptsI are significantly upregulated and those of hprK are significantly downregulated in response to copper ion-induced stress. Overexpression of ptsI significantly increases bacterial growth in vitro, while overexpression of hprK leads to a decrease in growth. We further demonstrate that DegU directly senses oxidative stress, downregulates ptsI transcription, and upregulates hprK transcription. Additionally, through an electrophoretic mobility shift assay, we demonstrate that DegU directly regulates the transcription of ptsI and hprK by binding to specific regions within their respective promoter sequences. Notably, the putative pivotal DegU binding sequence for ptsI is located from 38 to 68 base pairs upstream of the ptsH transcription start site (TSS), whereas for hprK, it is mapped from 36 to 124 base pairs upstream of the hprK TSS. In summary, we elucidate that DegU plays a significant role in suppressing carbohydrate uptake in response to oxidative stress through the direct regulation of ptsI and hprK.ImportanceUnderstanding the adaptive mechanisms employed by Listeria monocytogenes in harsh environments is of great significance. This study focuses on investigating the role of DegU in response to oxidative stress by examining global transcriptional profiles. The results highlight the noteworthy involvement of DegU in this stress response. Specifically, DegU acts as a direct sensor of oxidative stress, leading to the modulation of gene transcription. It downregulates ptsI transcription while it upregulates hprK transcription through direct binding to their promoters. Consequently, these regulatory actions impede bacterial growth, providing a defense mechanism against stress-induced damage. These findings gained from this study may have broader implications, serving as a reference for studying adaptive mechanisms in other pathogenic bacteria and aiding in the development of targeted strategies to control L. monocytogenes and ensure food safety.
Keywords: Listeria monocytogenes; PTS; hprK; orphan response regulator DegU; oxidative tolerance; ptsI.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
The DegU Orphan Response Regulator Contributes to Heat Stress Resistance in Listeria monocytogenes.Front Cell Infect Microbiol. 2021 Dec 13;11:761335. doi: 10.3389/fcimb.2021.761335. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34966695 Free PMC article.
-
Listeria monocytogenes GshF contributes to oxidative stress tolerance via regulation of the phosphoenolpyruvate-carbohydrate phosphotransferase system.Microbiol Spectr. 2023 Sep 5;11(5):e0236523. doi: 10.1128/spectrum.02365-23. Online ahead of print. Microbiol Spectr. 2023. PMID: 37668404 Free PMC article.
-
The DegU orphan response regulator of Listeria monocytogenes autorepresses its own synthesis and is required for bacterial motility, virulence and biofilm formation.Microbiology (Reading). 2008 Aug;154(Pt 8):2251-2264. doi: 10.1099/mic.0.2008/017590-0. Microbiology (Reading). 2008. PMID: 18667558
-
A pivotal role for the response regulator DegU in controlling multicellular behaviour.Microbiology (Reading). 2009 Jan;155(Pt 1):1-8. doi: 10.1099/mic.0.023903-0. Microbiology (Reading). 2009. PMID: 19118340 Review.
-
Transcription regulators potentially controlled by HPr kinase/phosphorylase in Gram-negative bacteria.J Mol Microbiol Biotechnol. 2003;5(4):206-15. doi: 10.1159/000071072. J Mol Microbiol Biotechnol. 2003. PMID: 12867744 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

