Glial plasticity at nervous system transition zones
- PMID: 37787575
- PMCID: PMC10562931
- DOI: 10.1242/bio.060037
Glial plasticity at nervous system transition zones
Abstract
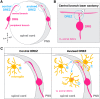
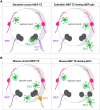
The central and peripheral nervous systems (CNS and PNS, respectively) are two separate yet connected domains characterized by molecularly distinct cellular components that communicate via specialized structures called transition zones to allow information to travel from the CNS to the periphery, and vice versa. Until recently, nervous system transition zones were thought to be selectively permeable only to axons, and the establishment of the territories occupied by glial cells at these complex regions remained poorly described and not well understood. Recent work now demonstrates that transition zones are occupied by dynamic glial cells and are precisely regulated over the course of nervous system development. This review highlights recent work on glial cell migration in and out of the spinal cord, at motor exit point (MEP) and dorsal root entry zone (DREZ) transition zones, in the physiological and diseased nervous systems. These cells include myelinating glia (oligodendrocyte lineage cells, Schwann cells and motor exit point glia), exit glia, perineurial cells that form the perineurium along spinal nerves, as well as professional and non-professional phagocytes (microglia and neural crest cells).
Keywords: Dorsal root entry zone; Glia; Migration; Motor exit point; Nervous system; Transition zone.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


Similar articles
-
Spinal cord precursors utilize neural crest cell mechanisms to generate hybrid peripheral myelinating glia.Elife. 2021 Feb 8;10:e64267. doi: 10.7554/eLife.64267. Elife. 2021. PMID: 33554855 Free PMC article.
-
Motor Exit Point (MEP) Glia: Novel Myelinating Glia That Bridge CNS and PNS Myelin.Front Cell Neurosci. 2018 Oct 2;12:333. doi: 10.3389/fncel.2018.00333. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30356886 Free PMC article. Review.
-
Contact-mediated inhibition between oligodendrocyte progenitor cells and motor exit point glia establishes the spinal cord transition zone.PLoS Biol. 2014 Sep 30;12(9):e1001961. doi: 10.1371/journal.pbio.1001961. eCollection 2014 Sep. PLoS Biol. 2014. PMID: 25268888 Free PMC article.
-
Perineurial Glial Plasticity and the Role of TGF-β in the Development of the Blood-Nerve Barrier.J Neurosci. 2017 May 3;37(18):4790-4807. doi: 10.1523/JNEUROSCI.2875-16.2017. Epub 2017 Apr 7. J Neurosci. 2017. PMID: 28389474 Free PMC article.
-
Peripheral glia diversity.J Anat. 2022 Nov;241(5):1219-1234. doi: 10.1111/joa.13484. Epub 2021 Jun 15. J Anat. 2022. PMID: 34131911 Free PMC article. Review.
Cited by
-
Neuroplasticity and Nervous System Recovery: Cellular Mechanisms, Therapeutic Advances, and Future Prospects.Brain Sci. 2025 Apr 15;15(4):400. doi: 10.3390/brainsci15040400. Brain Sci. 2025. PMID: 40309875 Free PMC article. Review.
-
The intriguing perineurial cells - an updated overview of their origin, structure, functions and implication in pathology.Rom J Morphol Embryol. 2024 Oct-Dec;65(4):567-574. doi: 10.47162/RJME.65.4.02. Rom J Morphol Embryol. 2024. PMID: 39957017 Free PMC article. Review.
References
-
- Bachelin, C., Zujovic, V., Buchet, D., Mallet, J. and Baron-Van Evercooren, A. (2009). Ectopic expression of polysialylated neural cell adhesion molecule in adult macaque Schwann cells promotes their migration and remyelination potential in the central nervous system. Brain 133, 406-420. 10.1093/brain/awp256 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

