Clinical Applications of Photon-counting CT: A Review of Pioneer Studies and a Glimpse into the Future
- PMID: 37787672
- PMCID: PMC10623209
- DOI: 10.1148/radiol.222432
Clinical Applications of Photon-counting CT: A Review of Pioneer Studies and a Glimpse into the Future
Abstract
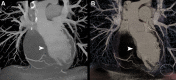
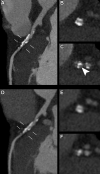
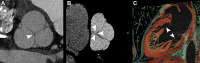
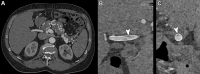
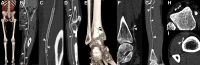
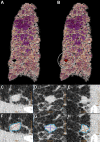
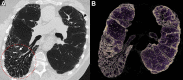
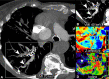
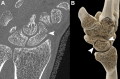
CT systems equipped with photon-counting detectors (PCDs), referred to as photon-counting CT (PCCT), are beginning to change imaging in several subspecialties, such as cardiac, vascular, thoracic, and musculoskeletal radiology. Evidence has been building in the literature underpinning the many advantages of PCCT for different clinical applications. These benefits derive from the distinct features of PCDs, which are made of semiconductor materials capable of converting photons directly into electric signal. PCCT advancements include, among the most important, improved spatial resolution, noise reduction, and spectral properties. PCCT spatial resolution on the order of 0.25 mm allows for the improved visualization of small structures (eg, small vessels, arterial walls, distal bronchi, and bone trabeculations) and their pathologies, as well as the identification of previously undetectable anomalies. In addition, blooming artifacts from calcifications, stents, and other dense structures are reduced. The benefits of the spectral capabilities of PCCT are broad and include reducing radiation and contrast material dose for patients. In addition, multiple types of information can be extracted from a single data set (ie, multiparametric imaging), including quantitative data often regarded as surrogates of functional information (eg, lung perfusion). PCCT also allows for a novel type of CT imaging, K-edge imaging. This technique, combined with new contrast materials specifically designed for this modality, opens the door to new applications for imaging in the future.
© RSNA, 2023.
Conflict of interest statement
Figures


References
-
- Cavallo AU , Patterson AJ , Thomas R , et al. . Low dose contrast CT for transcatheter aortic valve replacement assessment: results from the prospective SPECTACULAR study (spectral CT assessment prior to TAVR) . J Cardiovasc Comput Tomogr 2020. ; 14 ( 1 ): 68 – 74 . - PubMed
-
- Bae K , Jeon KN , Cho SB , et al. . Improved opacification of a suboptimally enhanced pulmonary artery in chest CT: experience using a dual-layer detector spectral CT . AJR Am J Roentgenol 2018. ; 210 ( 4 ): 734 – 741 . - PubMed
-
- Si-Mohamed S , Dupuis N , Tatard-Leitman V , et al. . Virtual versus true non-contrast dual-energy CT imaging for the diagnosis of aortic intramural hematoma . Eur Radiol 2019. ; 29 ( 12 ): 6762 – 6771 . - PubMed
-
- Johnson TRCC . Dual-energy CT: general principles . AJR Am J Roentgenol 2012. ; 199 ( 5 Suppl ): S3 – S8 . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

