B cell-intrinsic Myd88 regulates disease progression in murine lupus
- PMID: 37787782
- PMCID: PMC10541815
- DOI: 10.1084/jem.20230263
B cell-intrinsic Myd88 regulates disease progression in murine lupus
Abstract
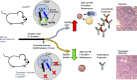
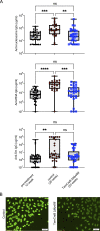
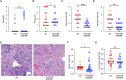
Nucleic acid-specific Toll-like receptors (TLRs) have been implicated in promoting disease pathogenesis in systemic lupus erythematosus (SLE). Whether such TLRs mediate disease onset, progression, or both remains undefined; yet the answer to this question has important therapeutic implications. MyD88 is an essential adaptor that acts downstream of IL-1 family receptors and most TLRs. Both global and B cell-specific Myd88 deficiency ameliorated disease in lupus-prone mice when constitutively deleted. To address whether Myd88 was needed to sustain ongoing disease, we induced B cell-specific deletion of Myd88 after disease onset in MRL.Faslpr mice using an inducible Cre recombinase. B cell-specific deletion of Myd88 starting after disease onset resulted in ameliorated glomerulonephritis and interstitial inflammation. Additionally, treated mice had reduced autoantibody formation and an altered B cell compartment with reduced ABC and plasmablast numbers. These experiments demonstrate the role of MyD88 in B cells to sustain disease in murine lupus. Therefore, targeting MyD88 or its upstream activators may be a viable therapeutic option in SLE.
© 2023 Tilstra et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures





References
-
- Alexander, T., Sarfert R., Klotsche J., Kühl A.A., Rubbert-Roth A., Lorenz H.M., Rech J., Hoyer B.F., Cheng Q., Waka A., et al. . 2015. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann. Rheum. Dis. 74:1474–1478. 10.1136/annrheumdis-2014-206016 - DOI - PMC - PubMed
-
- Christensen, S.R., Shupe J., Nickerson K., Kashgarian M., Flavell R.A., and Shlomchik M.J.. 2006. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 25:417–428. 10.1016/j.immuni.2006.07.013 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

