Remote Ischemic Conditioning for Acute Stroke: The RESIST Randomized Clinical Trial
- PMID: 37787796
- PMCID: PMC10548297
- DOI: 10.1001/jama.2023.16893
Remote Ischemic Conditioning for Acute Stroke: The RESIST Randomized Clinical Trial
Abstract
Importance: Despite some promising preclinical and clinical data, it remains uncertain whether remote ischemic conditioning (RIC) with transient cycles of limb ischemia and reperfusion is an effective treatment for acute stroke.
Objective: To evaluate the effect of RIC when initiated in the prehospital setting and continued in the hospital on functional outcome in patients with acute stroke.
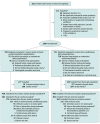
Design, setting, and participants: This was a randomized clinical trial conducted at 4 stroke centers in Denmark that included 1500 patients with prehospital stroke symptoms for less than 4 hours (enrolled March 16, 2018, to November 11, 2022; final follow-up, February 3, 2023).
Intervention: The intervention was delivered using an inflatable cuff on 1 upper extremity (RIC cuff pressure, ≤200 mm Hg [n = 749] and sham cuff pressure, 20 mm Hg [n = 751]). Each treatment application consisted of 5 cycles of 5 minutes of cuff inflation followed by 5 minutes of cuff deflation. Treatment was started in the ambulance and repeated at least once in the hospital and then twice daily for 7 days among a subset of participants.
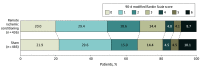
Main outcomes and measures: The primary end point was improvement in functional outcome measured as a shift across the modified Rankin Scale (mRS) score (range, 0 [no symptoms] to 6 [death]) at 90 days in the target population with a final diagnosis of ischemic or hemorrhagic stroke.
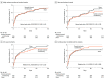
Results: Among 1500 patients who were randomized (median age, 71 years; 591 women [41%]), 1433 (96%) completed the trial. Of these, 149 patients (10%) were diagnosed with transient ischemic attack and 382 (27%) with a stroke mimic. In the remaining 902 patients with a target diagnosis of stroke (737 [82%] with ischemic stroke and 165 [18%] with intracerebral hemorrhage), 436 underwent RIC and 466 sham treatment. The median mRS score at 90 days was 2 (IQR, 1-3) in the RIC group and 1 (IQR, 1-3) in the sham group. RIC treatment was not significantly associated with improved functional outcome at 90 days (odds ratio [OR], 0.95; 95% CI, 0.75 to 1.20, P = .67; absolute difference in median mRS score, -1; -1.7 to -0.25). In all randomized patients, there were no significant differences in the number of serious adverse events: 169 patients (23.7%) in the RIC group with 1 or more serious adverse events vs 175 patients (24.3%) in the sham group (OR, 0.97; 95% CI, 0.85 to 1.11; P = .68). Upper extremity pain during treatment and/or skin petechia occurred in 54 (7.2%) in the RIC group and 11 (1.5%) in the sham group.
Conclusions and relevance: RIC initiated in the prehospital setting and continued in the hospital did not significantly improve functional outcome at 90 days in patients with acute stroke.
Trial registration: ClinicalTrials.gov Identifier: NCT03481777.
Conflict of interest statement
Figures