Proprotein convertase subtisilin/kexin 9 levels decline with hepatitis C virus therapy in people with HIV/hepatitis C virus and correlate with inflammation
- PMID: 37788081
- PMCID: PMC10841736
- DOI: 10.1097/QAD.0000000000003739
Proprotein convertase subtisilin/kexin 9 levels decline with hepatitis C virus therapy in people with HIV/hepatitis C virus and correlate with inflammation
Abstract
Background: Proprotein convertase subtisilin/kexin 9 (PCSK9) raises low-density lipoprotein cholesterol (LDL-C) levels and is associated with inflammation, which is elevated in HIV and hepatitis C virus (HCV) infection. We compared PCSK9 levels in people with co-occurring HIV and HCV (HIV/HCV) vs. HIV alone, and evaluated the impact of HCV direct-acting antiviral (DAA) therapy on PCSK9.
Design: A prospective, observational cohort study.
Methods: Thirty-five adults with HIV/HCV and 37 with HIV alone were evaluated, all with HIV virologic suppression and without documented cardiovascular disease. Circulating PCSK9 and inflammatory biomarkers were measured at baseline and following HCV treatment or at week 52 (for HIV alone) and compared using Wilcoxon tests and Spearman correlations.
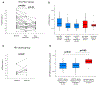
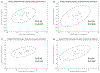
Results: At baseline, PCSK9 trended higher in HIV/HCV vs. HIV alone (307 vs. 284 ng/ml, P = 0.06). Twenty-nine participants with HIV/HCV completed DAA therapy with sustained virologic response. PCSK9 declined from baseline to posttreatment 1 (median 7.3 weeks after end of therapy [EOT]) and posttreatment 2 (median 43.5 weeks after EOT), reaching levels similar to HIV alone; median within-person reduction was -60.5 ng/ml ( P = 0.003) and -55.6 ng/ml ( P = 0.02), respectively. Decline in PCSK9 correlated with decline in soluble (s)E-selectin and sCD163 ( r = 0.64, P = 0.002; r = 0.58, P = 0.008, respectively), but not with changes in LDL-C or other biomarkers. No significant change in PCSK9 occurred in the HIV alone group over 52 weeks.
Conclusion: PCSK9 declined with DAA therapy in participants with HIV/HCV, correlating with declines in several inflammatory biomarkers but not LDL-C. Elevated PCSK9 with HCV may be linked to particular HCV-associated inflammatory pathways more so than cholesterol homeostasis.
Copyright © 2023 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
KWC has received research funding to the institution from Merck Sharp & Dohme and Amgen and served as a consultant for Pardes Biosciences. CNBM serves as a consultant to iRhythm and SHL Telemedicine, and receives support from the Barbra Streisand Women’s Cardiovascular Research and Education Program, The Linda Joy Pollin Women’s Heart Health Program, and the Erika Glazer Women’s Heart Health Project, Cedars-Sinai Medical Center, Los Angeles, California. KLN receives grant support from the Veterans Health Administration (CX001901, CX002208) and the National Institutes of Health (R01HL148182, R01HL127153). ESD received research support from Gilead and ViiV and is consultant for Gilead, Merck and ViiV. JEL receives research support from Gilead Sciences and is a consultant to Theratechnologies, and receives grant support from the National Institutes of Health (R01DK126042.)
Figures

Similar articles
-
HIV and Hepatitis C-Coinfected Patients Have Lower Low-Density Lipoprotein Cholesterol Despite Higher Proprotein Convertase Subtilisin Kexin 9 (PCSK9): An Apparent "PCSK9-Lipid Paradox".J Am Heart Assoc. 2016 Apr 29;5(5):e002683. doi: 10.1161/JAHA.115.002683. J Am Heart Assoc. 2016. PMID: 27130349 Free PMC article.
-
Treatment-Induced Viral Cure of Hepatitis C Virus-Infected Patients Involves a Dynamic Interplay among three Important Molecular Players in Lipid Homeostasis: Circulating microRNA (miR)-24, miR-223, and Proprotein Convertase Subtilisin/Kexin Type 9.EBioMedicine. 2017 Sep;23:68-78. doi: 10.1016/j.ebiom.2017.08.020. Epub 2017 Aug 24. EBioMedicine. 2017. PMID: 28864162 Free PMC article.
-
PCSK9, apolipoprotein E and lipoviral particles in chronic hepatitis C genotype 3: evidence for genotype-specific regulation of lipoprotein metabolism.J Hepatol. 2015 Apr;62(4):763-70. doi: 10.1016/j.jhep.2014.11.016. Epub 2014 Nov 21. J Hepatol. 2015. PMID: 25463543
-
Hepatitis C virus and proprotein convertase subtilisin/kexin type 9: a detrimental interaction to increase viral infectivity and disrupt lipid metabolism.J Cell Mol Med. 2017 Dec;21(12):3150-3161. doi: 10.1111/jcmm.13273. Epub 2017 Jul 18. J Cell Mol Med. 2017. PMID: 28722331 Free PMC article. Review.
-
Potential use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibition and prevention method in viral infection.Microb Cell Fact. 2024 Mar 25;23(1):90. doi: 10.1186/s12934-024-02355-8. Microb Cell Fact. 2024. Retraction in: Microb Cell Fact. 2024 Nov 25;23(1):316. doi: 10.1186/s12934-024-02594-9. PMID: 38528584 Free PMC article. Retracted. Review.
Cited by
-
Inflammatory and Immune Mechanisms for Atherosclerotic Cardiovascular Disease in HIV.Int J Mol Sci. 2024 Jul 1;25(13):7266. doi: 10.3390/ijms25137266. Int J Mol Sci. 2024. PMID: 39000373 Free PMC article. Review.
References
-
- Seidah NG, Abifadel M, Prost S, Boileau C, Prat A. The Proprotein Convertases in Hypercholesterolemia and Cardiovascular Diseases: Emphasis on Proprotein Convertase Subtilisin/Kexin 9. Pharmacol Rev 2017; 69(1):33–52. - PubMed
-
- Karagiannis AD, Liu M, Toth PP, Zhao S, Agrawal DK, Libby P, et al. Pleiotropic Anti-atherosclerotic Effects of PCSK9 InhibitorsFrom Molecular Biology to Clinical Translation. Curr Atheroscler Rep 2018; 20(4):20. - PubMed
-
- Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med 2017; 376(18):1713–1722. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

