Radiotracer Development for Fungal-Specific Imaging: Past, Present, and Future
- PMID: 37788500
- PMCID: PMC10547453
- DOI: 10.1093/infdis/jiad067
Radiotracer Development for Fungal-Specific Imaging: Past, Present, and Future
Abstract
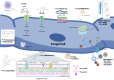
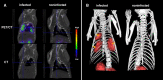
Invasive fungal infections have become a major challenge for public health, mainly due to the growing numbers of immunocompromised patients, with high morbidity and mortality. Currently, conventional imaging modalities such as computed tomography and magnetic resonance imaging contribute largely to the noninvasive diagnosis and treatment evaluation of those infections. These techniques, however, often fall short when a fast, noninvasive and specific diagnosis of fungal infection is necessary. Molecular imaging, especially using nuclear medicine-based techniques, aims to develop fungal-specific radiotracers that can be tested in preclinical models and eventually translated to human applications. In the last few decades, multiple radioligands have been developed and tested as potential fungal-specific tracers. These include radiolabeled peptides, antifungal drugs, siderophores, fungal-specific antibodies, and sugars. In this review, we provide an overview of the pros and cons of the available radiotracers. We also address the future prospects of fungal-specific imaging.
Keywords: PET; immunoPET; invasive fungal infection; radionuclide imaging; siderophores.
Published by Oxford University Press on behalf of Infectious Diseases Society of America 2023.
Conflict of interest statement
Conflict of interest. D. A. H. is first named inventor for pending patent application US2020/044446 on radiolabeled sugars for imaging of fungal infections, filed by the National Institutes of Health. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



Similar articles
-
Imaging of Invasive Fungal Infections- The Role of PET/CT.Semin Nucl Med. 2023 Jan;53(1):57-69. doi: 10.1053/j.semnuclmed.2022.07.003. Epub 2022 Aug 4. Semin Nucl Med. 2023. PMID: 35933165 Review.
-
Recent Advancements in Radiopharmaceuticals for Infection Imaging.Methods Mol Biol. 2024;2813:205-217. doi: 10.1007/978-1-0716-3890-3_14. Methods Mol Biol. 2024. PMID: 38888780 Review.
-
PET Imaging of Active Invasive Fungal Infections with d-[5-11C]-Glutamine.ACS Infect Dis. 2022 Aug 12;8(8):1663-1673. doi: 10.1021/acsinfecdis.2c00249. Epub 2022 Jul 22. ACS Infect Dis. 2022. PMID: 35869564
-
Magnetic resonance imaging (MRI) for the assessment of myocardial viability: an evidence-based analysis.Ont Health Technol Assess Ser. 2010;10(15):1-45. Epub 2010 Jul 1. Ont Health Technol Assess Ser. 2010. PMID: 23074392 Free PMC article.
-
FDG PET/CT imaging in detecting and guiding management of invasive fungal infections: a retrospective comparison to conventional CT imaging.Eur J Nucl Med Mol Imaging. 2019 Jan;46(1):166-173. doi: 10.1007/s00259-018-4062-8. Epub 2018 Jun 7. Eur J Nucl Med Mol Imaging. 2019. PMID: 29882160
Cited by
-
Refractory versus resistant invasive aspergillosis.J Antimicrob Chemother. 2025 Mar 14;80(Supplement_1):i9-i16. doi: 10.1093/jac/dkaf003. J Antimicrob Chemother. 2025. PMID: 40085537 Free PMC article. Review.
-
PET imaging of Aspergillus infection using Zirconium-89 labeled anti-β-glucan antibody fragments.Eur J Nucl Med Mol Imaging. 2024 Sep;51(11):3223-3234. doi: 10.1007/s00259-024-06760-4. Epub 2024 May 24. Eur J Nucl Med Mol Imaging. 2024. PMID: 38787397 Free PMC article.
-
Is Pulmonary Mycoses Shadowed by Tuberculosis? Mandate to Hit the Bull's Eye-An Indian Perspective.Pathogens. 2025 Apr 30;14(5):435. doi: 10.3390/pathogens14050435. Pathogens. 2025. PMID: 40430764 Free PMC article. Review.
-
Nuclear Medicine Imaging Tools in Fever of Unknown Origin: Time for a Revisit and Appropriate Use Criteria.Clin Infect Dis. 2024 May 15;78(5):1148-1153. doi: 10.1093/cid/ciae115. Clin Infect Dis. 2024. PMID: 38441140 Free PMC article.
-
18F-Fluorodeoxysorbitol PET for noninvasive detection of invasive mold infections: preclinical and first-in-human studies.Nat Commun. 2025 Jul 10;16(1):6395. doi: 10.1038/s41467-025-61700-6. Nat Commun. 2025. PMID: 40640182 Free PMC article.
References
-
- Zhao C, Mendive-Tapia L, Vendrell M. Fluorescent peptides for imaging of fungal cells. Arch Biochem Biophys 2019; 661:187–95. - PubMed
-
- Thompson GR III, Young JH. Aspergillus infections. N Engl J Med 2021; 385:1496–509. - PubMed
-
- Antimicrobial Resistance Division CoNTD, Global Coordination and Partnership, World Health Organization (WHO). Fungal priority pathogens list to guide research, development and public health action. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO
-
- Vermeulen E, Lagrou K, Verweij PE. Azole resistance in Aspergillus fumigatus: a growing public health concern. Curr Opin Infect Dis 2013; 26:493–500. - PubMed
-
- Kontoyiannis DP, Sumoza D, Tarrand J, Bodey GP, Storey R, Raad II. Significance of aspergillemia in patients with cancer: a 10-year study. Clin Infect Dis 2000; 31:188–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

