HLA-DPA1*02:01~B1*01:01 is a risk haplotype for primary sclerosing cholangitis mediating activation of NKp44+ NK cells
- PMID: 37788895
- PMCID: PMC10850656
- DOI: 10.1136/gutjnl-2023-329524
HLA-DPA1*02:01~B1*01:01 is a risk haplotype for primary sclerosing cholangitis mediating activation of NKp44+ NK cells
Abstract
Objective: Primary sclerosing cholangitis (PSC) is characterised by bile duct strictures and progressive liver disease, eventually requiring liver transplantation. Although the pathogenesis of PSC remains incompletely understood, strong associations with HLA-class II haplotypes have been described. As specific HLA-DP molecules can bind the activating NK-cell receptor NKp44, we investigated the role of HLA-DP/NKp44-interactions in PSC.
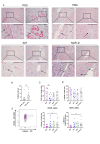
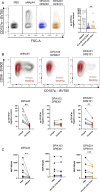
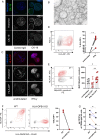
Design: Liver tissue, intrahepatic and peripheral blood lymphocytes of individuals with PSC and control individuals were characterised using flow cytometry, immunohistochemical and immunofluorescence analyses. HLA-DPA1 and HLA-DPB1 imputation and association analyses were performed in 3408 individuals with PSC and 34 213 controls. NK cell activation on NKp44/HLA-DP interactions was assessed in vitro using plate-bound HLA-DP molecules and HLA-DPB wildtype versus knock-out human cholangiocyte organoids.
Results: NKp44+NK cells were enriched in livers, and intrahepatic bile ducts of individuals with PSC showed higher expression of HLA-DP. HLA-DP haplotype analysis revealed a highly elevated PSC risk for HLA-DPA1*02:01~B1*01:01 (OR 1.99, p=6.7×10-50). Primary NKp44+NK cells exhibited significantly higher degranulation in response to plate-bound HLA-DPA1*02:01-DPB1*01:01 compared with control HLA-DP molecules, which were inhibited by anti-NKp44-blocking. Human cholangiocyte organoids expressing HLA-DPA1*02:01-DPB1*01:01 after IFN-γ-exposure demonstrated significantly increased binding to NKp44-Fc constructs compared with unstimulated controls. Importantly, HLA-DPA1*02:01-DPB1*01:01-expressing organoids increased degranulation of NKp44+NK cells compared with HLA-DPB1-KO organoids.
Conclusion: Our studies identify a novel PSC risk haplotype HLA-DP A1*02:01~DPB1*01:01 and provide clinical and functional data implicating NKp44+NK cells that recognise HLA-DPA1*02:01-DPB1*01:01 expressed on cholangiocytes in PSC pathogenesis.
Keywords: AUTOIMMUNE BILIARY DISEASE; IMMUNE-MEDIATED LIVER DAMAGE; IMMUNOGENETICS; IMMUNOLOGY IN HEPATOLOGY; PRIMARY SCLEROSING CHOLANGITIS.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
