Participate CP 2: optimising participation in physically active leisure for children with cerebral palsy - protocol for a phase III randomised controlled trial
- PMID: 37788925
- PMCID: PMC10551958
- DOI: 10.1136/bmjopen-2023-075570
Participate CP 2: optimising participation in physically active leisure for children with cerebral palsy - protocol for a phase III randomised controlled trial
Abstract
Introduction: Children with cerebral palsy (CP) participate less in physical activities and have increased sedentary behaviour compared with typically developing peers. Participate CP is a participation-focused therapy intervention for children with CP with demonstrated efficacy in a phase II randomised controlled trial (RCT) to increase perceived performance of physical activity participation goals. This study will test the effectiveness of Participate CP in a multisite phase III RCT.
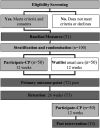
Methods and analysis: One hundred children with CP, aged 8-14 years, classified Gross Motor Function Classification System levels I-IV will be randomised to either (1) receive Participate CP once/week for 1 hour for 12 weeks, or (2) waitlist control, usual care group. The waitlist group will then receive Participate CP following the 26-week retention time point. Outcomes will be assessed at baseline, 12 weeks and then 26 weeks post baseline. The primary outcomes are (1) self-reported participation goal performance on the Canadian Occupational Performance Measure at 12 weeks and (2) daily time in moderate-to-vigorous physical activity. Secondary outcomes include home and community participation frequency, involvement and environmental supportiveness, contextual barriers to participation, quality of life, intrinsic motivation for physical activities, child perception of an autonomy-supportive climate for physical activities and physical literacy at 12 and 26 weeks post study entry.
Ethics and dissemination: The Children's Health Queensland Hospital and Health Service, The University of Queensland and the New Zealand Health and Disability Ethics Committees have approved this study. Findings will be disseminated in peer-reviewed journals and conference presentations.
Trial registration number: ACTRN12618000206224.
Keywords: clinical trial; developmental neurology & neurodisability; paediatric neurology; physical therapy modalities.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Study protocol for Active Start Active Future: a randomised control trial of an early behaviour-change intervention targeting physical activity participation and sedentary behaviour in young children with cerebral palsy living in South East Queensland, Australia.BMJ Open. 2025 May 19;15(5):e087697. doi: 10.1136/bmjopen-2024-087697. BMJ Open. 2025. PMID: 40389315 Free PMC article.
-
ParticiPAte CP: a protocol of a randomised waitlist controlled trial of a motivational and behaviour change therapy intervention to increase physical activity through meaningful participation in children with cerebral palsy.BMJ Open. 2017 Aug 7;7(8):e015918. doi: 10.1136/bmjopen-2017-015918. BMJ Open. 2017. PMID: 28790038 Free PMC article. Clinical Trial.
-
ACTIVE STRIDES-CP: protocol for a randomised trial of intensive rehabilitation (combined intensive gait and cycling training) for children with moderate-to-severe bilateral cerebral palsy.BMJ Open. 2023 Mar 29;13(3):e068774. doi: 10.1136/bmjopen-2022-068774. BMJ Open. 2023. PMID: 36990490 Free PMC article.
-
Constraint-induced movement therapy in children with unilateral cerebral palsy.Cochrane Database Syst Rev. 2019 Apr 1;4(4):CD004149. doi: 10.1002/14651858.CD004149.pub3. Cochrane Database Syst Rev. 2019. PMID: 30932166 Free PMC article.
-
Bisphosphonate use in children with cerebral palsy.Cochrane Database Syst Rev. 2021 Jul 5;7(7):CD012756. doi: 10.1002/14651858.CD012756.pub2. Cochrane Database Syst Rev. 2021. PMID: 34224134 Free PMC article.
Cited by
-
SPIRIT 2025 explanation and elaboration: updated guideline for protocols of randomised trials.BMJ. 2025 Apr 28;389:e081660. doi: 10.1136/bmj-2024-081660. BMJ. 2025. PMID: 40294956 Free PMC article.
-
Study protocol for Active Start Active Future: a randomised control trial of an early behaviour-change intervention targeting physical activity participation and sedentary behaviour in young children with cerebral palsy living in South East Queensland, Australia.BMJ Open. 2025 May 19;15(5):e087697. doi: 10.1136/bmjopen-2024-087697. BMJ Open. 2025. PMID: 40389315 Free PMC article.
References
-
- Access Economics . The Economic Impact of Cerebral Palsy in Australia in 2007. 2008.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous