Comparing persistence of new biologics to conventional anti-TNF alphas in adult patients with inflammatory bowel disease: a systematic review and meta-analysis protocol
- PMID: 37788929
- PMCID: PMC10551959
- DOI: 10.1136/bmjopen-2023-073071
Comparing persistence of new biologics to conventional anti-TNF alphas in adult patients with inflammatory bowel disease: a systematic review and meta-analysis protocol
Abstract
Background: Biological therapy is a cornerstone of managing moderate-to-severe inflammatory bowel disease (IBD), ulcerative colitis (UC) and Crohn's disease (CD). New biologics have been evolving over the past 20 years and selection of an agent remains challenging.Drug persistence measures the duration of time from initiation to discontinuation of a therapy, which can be a surrogate marker of drug tolerance and efficacy.
Objectives: The study aimed to compare drug persistence of new generation biologics for the treatment of UC and CD (vedolizumab, ustekinumab, certolizumab, tofacitinib, natalizumab and golimumab) with conventional anti-tumor necroisis factor alphas (anti-TNF alphas) (adalimumab and infliximab) in adult patients with IBD. Results of the study may provide guidance on the preferred first and subsequent lines of biological treatments in patients with IBD.
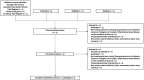
Methods and analysis: Search via electronic databases including EMBASE, MEDLINE, PubMed and clinical trial databases will be conducted on 10 March 2023 with eligible studies included from inception of 2017 to 2023. The primary outcomes are 1-year persistence of individual biologics with comparison of new biologics versus conventional anti-TNF alphas. A meta-analysis will be conducted using Review Manager V.5 and outcome will be presented as relative risk. Heterogeneity will be assessed with forest plot, χ2 and I2, followed with sensitivity analysis and subgroup analysis. Finally, the Grading of Recommendations Assessment, Development and Evaluation system will be used to assess the quality of evidence.
Ethics and dissemination: Ethical approval is not required as no private information of participants will be used. Results of the present study will be disseminated in a peer-reviewed journal or conference presentation.
Prospero registration number: CRD42023392236.
Keywords: adult gastroenterology; clinical pharmacology; inflammatory bowel disease; therapeutics.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RWL is an advisory board member of AbbVie, Aspen, BMS, Celgene, Celltrion, Chiesi, Ferring, Glutagen, Hospira, Janssen, Lilly, MSD, Novartis, Pfizer, Prometheus Biosciences, Takeda; research grant recipient of Celltrion, Shire, Janssen, Takeda, Gastroenterological Society of Australia, NHMRC, Gutsy Group, Pfizer and Joanna Tiddy grants University of Sydney. AP is an advisory board member of AbbVie and received speaker fees from AbbVie and Takeda.
Figures
Similar articles
-
Efficacy of Biologic Drugs in Short-Duration Versus Long-Duration Inflammatory Bowel Disease: A Systematic Review and an Individual-Patient Data Meta-Analysis of Randomized Controlled Trials.Gastroenterology. 2022 Feb;162(2):482-494. doi: 10.1053/j.gastro.2021.10.037. Epub 2021 Oct 29. Gastroenterology. 2022. PMID: 34757139
-
Real-World Persistence of Successive Biologics in Patients With Inflammatory Bowel Disease: Findings From ROTARY.Inflamm Bowel Dis. 2024 Oct 3;30(10):1776-1787. doi: 10.1093/ibd/izad245. Inflamm Bowel Dis. 2024. PMID: 37921344 Free PMC article.
-
Biologic Use Patterns and Predictors for Non-persistence and Switching of Biologics in Patients with Inflammatory Bowel Disease: A Nationwide Population-Based Study.Dig Dis Sci. 2020 May;65(5):1436-1444. doi: 10.1007/s10620-019-05867-1. Epub 2019 Nov 1. Dig Dis Sci. 2020. PMID: 31677070
-
Efficacy of biological drugs in short-duration versus long-duration inflammatory bowel disease: a protocol for a systematic review and an individual-patient level meta-analysis of randomised controlled trials.BMJ Open. 2019 Jan 25;9(1):e024222. doi: 10.1136/bmjopen-2018-024222. BMJ Open. 2019. PMID: 30782731 Free PMC article.
-
Management of inflammatory bowel disease with infliximab and other anti-tumor necrosis factor alpha therapies.BioDrugs. 2010 Dec 14;24 Suppl 1:3-14. doi: 10.2165/11586290-000000000-00000. BioDrugs. 2010. PMID: 21175228 Review.
Cited by
-
Persistence of advanced therapies in patients with inflammatory bowel disease: retrospective cohort study using a large healthcare claims database in Japan.Intest Res. 2025 Jul;23(3):358-371. doi: 10.5217/ir.2024.00118. Epub 2025 Jan 2. Intest Res. 2025. PMID: 39701922 Free PMC article.
References
-
- PricewaterhouseCoopers Australia (PwC) . Improving inflammatory bowel disease care across Australia. 2013. Available: https://silo.tips/download/improving-inflammatory-bowel-disease-care-acr...
-
- Australian Government . Department of health and aged care. The pharmaceutical benefits scheme. 2022. Available: https://www.pbs.gov.au/browse/medicine-listing
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous