The uncertain role of substandard and falsified medicines in the emergence and spread of antimicrobial resistance
- PMID: 37788991
- PMCID: PMC10547756
- DOI: 10.1038/s41467-023-41542-w
The uncertain role of substandard and falsified medicines in the emergence and spread of antimicrobial resistance
Abstract
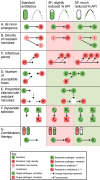
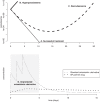
Approximately 10% of antimicrobials used by humans in low- and middle-income countries are estimated to be substandard or falsified. In addition to their negative impact on morbidity and mortality, they may also be important drivers of antimicrobial resistance. Despite such concerns, our understanding of this relationship remains rudimentary. Substandard and falsified medicines have the potential to either increase or decrease levels of resistance, and here we discuss a range of mechanisms that could drive these changes. Understanding these effects and their relative importance will require an improved understanding of how different drug exposures affect the emergence and spread of resistance and of how the percentage of active pharmaceutical ingredients in substandard and falsified medicines is temporally and spatially distributed.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- World Health Organization. WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products (World Health Organization, 2017).
-
- World Health Organization. A Study on the Public Health and Socioeconomic Impact of Substandard and Falsified Medical Products (World Health Organization, 2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

