Pleconaril and ribavirin in new-onset type 1 diabetes: a phase 2 randomized trial
- PMID: 37789144
- PMCID: PMC10667091
- DOI: 10.1038/s41591-023-02576-1
Pleconaril and ribavirin in new-onset type 1 diabetes: a phase 2 randomized trial
Abstract
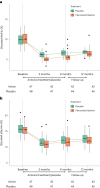
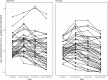
Previous studies showed a low-grade enterovirus infection in the pancreatic islets of patients with newly diagnosed type 1 diabetes (T1D). In the Diabetes Virus Detection (DiViD) Intervention, a phase 2, placebo-controlled, randomized, parallel group, double-blind trial, 96 children and adolescents (aged 6-15 years) with new-onset T1D received antiviral treatment with pleconaril and ribavirin (n = 47) or placebo (n = 49) for 6 months, with the aim of preserving β cell function. The primary endpoint was the mean stimulated C-peptide area under the curve (AUC) 12 months after the initiation of treatment (less than 3 weeks after diagnosis) using a mixed linear model. The model used longitudinal log-transformed serum C-peptide AUCs at baseline, at 3 months, 6 months and 1 year. The primary endpoint was met with the serum C-peptide AUC being higher in the pleconaril and ribavirin treatment group compared to the placebo group at 12 months (average marginal effect = 0.057 in the linear mixed model; 95% confidence interval = 0.004-0.11, P = 0.037). The treatment was well tolerated. The results show that antiviral treatment may preserve residual insulin production in children and adolescent with new-onset T1D. This provides a rationale for further evaluating antiviral strategies in the prevention and treatment of T1D. European Union Drug Regulating Authorities Clinical Trials identifier: 2015-003350-41 .
© 2023. The Author(s).
Conflict of interest statement
H.H. is a shareholder and member of the board of Vactech Ltd, which develops vaccines against picornaviruses. N.L. and J.W. are employees of Apodemus/Curovir AB, which own the rights to the commercialization of the results of this trial. Curovir AB is developing antiviral drugs for enterovirus-induced diseases. The other authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

