Extrusion-based 3D printing of osteoinductive scaffolds with a spongiosa-inspired structure
- PMID: 37790253
- PMCID: PMC10544914
- DOI: 10.3389/fbioe.2023.1268049
Extrusion-based 3D printing of osteoinductive scaffolds with a spongiosa-inspired structure
Abstract
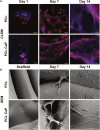
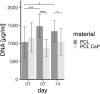
Critical-sized bone defects resulting from trauma, inflammation, and tumor resections are individual in their size and shape. Implants for the treatment of such defects have to consider biomechanical and biomedical factors, as well as the individual conditions within the implantation site. In this context, 3D printing technologies offer new possibilities to design and produce patient-specific implants reflecting the outer shape and internal structure of the replaced bone tissue. The selection or modification of materials used in 3D printing enables the adaption of the implant, by enhancing the osteoinductive or biomechanical properties. In this study, scaffolds with bone spongiosa-inspired structure for extrusion-based 3D printing were generated. The computer aided design process resulted in an up scaled and simplified version of the bone spongiosa. To enhance the osteoinductive properties of the 3D printed construct, polycaprolactone (PCL) was combined with 20% (wt) calcium phosphate nano powder (CaP). The implants were designed in form of a ring structure and revealed an irregular and interconnected porous structure with a calculated porosity of 35.2% and a compression strength within the range of the natural cancellous bone. The implants were assessed in terms of biocompatibility and osteoinductivity using the osteosarcoma cell line MG63 and patient-derived mesenchymal stem cells in selected experiments. Cell growth and differentiation over 14 days were monitored using confocal laser scanning microscopy, scanning electron microscopy, deoxyribonucleic acid (DNA) quantification, gene expression analysis, and quantitative assessment of calcification. MG63 cells and human mesenchymal stem cells (hMSC) adhered to the printed implants and revealed a typical elongated morphology as indicated by microscopy. Using DNA quantification, no differences for PCL or PCL-CaP in the initial adhesion of MG63 cells were observed, while the PCL-based scaffolds favored cell proliferation in the early phases of culture up to 7 days. In contrast, on PCL-CaP, cell proliferation for MG63 cells was not evident, while data from PCR and the levels of calcification, or alkaline phosphatase activity, indicated osteogenic differentiation within the PCL-CaP constructs over time. For hMSC, the highest levels in the total calcium content were observed for the PCL-CaP constructs, thus underlining the osteoinductive properties.
Keywords: 3D printing; PCL; bone implant; calcium phosphate; critical-sized bone defect; extrusion-based printing; osteogenic differentiation; scaffold.
Copyright © 2023 Kühl, Gorb, Kern, Klüter, Kühl, Seekamp and Fuchs.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures







References
-
- Abbasi N., Hamlet S., Love R. M., Nguyen N-T. (2020). Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 5 (1), 1–9. 10.1016/j.jsamd.2020.01.007 - DOI
-
- Alvarez K., Nakajima H. (2009). Metallic scaffolds for bone regeneration. Materials 2 (3), 790–832. 10.3390/ma2030790 - DOI
-
- Arakawa C. K., DeForest C. A. (2017). “Chapter 19 - polymer design and development,” in Biology and engineering of stem cell niches. Editors Vishwakarma A., Karp J. M. (Boston: Academic Press; ), 295–314.
LinkOut - more resources
Full Text Sources
Miscellaneous

