Immunogenicity of COVID-19 vaccines and their effect on HIV reservoir in older people with HIV
- PMID: 37790281
- PMCID: PMC10542941
- DOI: 10.1016/j.isci.2023.107915
Immunogenicity of COVID-19 vaccines and their effect on HIV reservoir in older people with HIV
Abstract
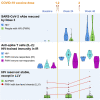
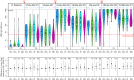
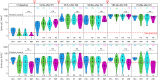
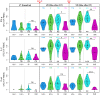
Older individuals and people with HIV (PWH) were prioritized for COVID-19 vaccination, yet comprehensive studies of the immunogenicity of these vaccines and their effects on HIV reservoirs are not available. Our study on 68 PWH and 23 HIV-negative participants aged 55 and older post-three vaccine doses showed equally strong anti-spike IgG responses in serum and saliva through week 48 from baseline, while PWH salivary IgA responses were low. PWH had diminished live-virus neutralization responses after two vaccine doses, which were 'rescued' post-booster. Spike-specific T cell immunity was enhanced in PWH with normal CD4+ T cell count, suggesting Th1 imprinting. The frequency of detectable HIV viremia increased post-vaccination, but vaccines did not affect the size of the HIV reservoir in most PWH, except those with low-level viremia. Thus, older PWH require three doses of COVID-19 vaccine for maximum protection, while individuals with unsuppressed viremia should be monitored for adverse reactions from HIV reservoirs.
Keywords: Immunology; Virology.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures




Update of
-
Immunogenicity of COVID-19 vaccines and their effect on the HIV reservoir in older people with HIV.bioRxiv [Preprint]. 2023 Jun 15:2023.06.14.544834. doi: 10.1101/2023.06.14.544834. bioRxiv. 2023. Update in: iScience. 2023 Sep 14;26(10):107915. doi: 10.1016/j.isci.2023.107915. PMID: 37502977 Free PMC article. Updated. Preprint.
References
-
- Tesoriero J.M., Swain C.A.E., Pierce J.L., Zamboni L., Wu M., Holtgrave D.R., Gonzalez C.J., Udo T., Morne J.E., Hart-Malloy R., et al. COVID-19 Outcomes Among Persons Living With or Without Diagnosed HIV Infection in New York State. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.37069. - DOI - PMC - PubMed
-
- Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases South Africa. Davies M.-A., Hussey H., Ismail M., Morden E., Vundle Z., Zweigenthal V. Risk Factors for Coronavirus Disease 2019 (COVID-19) Death in a Population Cohort Study from the Western Cape Province, South Africa. Clin. Infect. Dis. 2021;73:e2005–e2015. doi: 10.1093/cid/ciaa1198. - DOI - PMC - PubMed
-
- Bhaskaran K., Rentsch C.T., MacKenna B., Schultze A., Mehrkar A., Bates C.J., Eggo R.M., Morton C.E., Bacon S.C.J., Inglesby P., et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet. HIV. 2021;8:e24–e32. doi: 10.1016/S2352-3018(20)30305-2. - DOI - PMC - PubMed
-
- Sigel K., Swartz T., Golden E., Paranjpe I., Somani S., Richter F., De Freitas J.K., Miotto R., Zhao S., Polak P., et al. Coronavirus 2019 and People Living With Human Immunodeficiency Virus: Outcomes for Hospitalized Patients in New York City. Clin. Infect. Dis. 2020;71:2933–2938. doi: 10.1093/cid/ciaa880. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

