This is a preprint.
Fibrinolytic-deficiencies predispose hosts to septicemia from a catheter-associated UTI
- PMID: 37790429
- PMCID: PMC10543281
- DOI: 10.21203/rs.3.rs-3263501/v1
Fibrinolytic-deficiencies predispose hosts to septicemia from a catheter-associated UTI
Update in
-
Fibrinolytic-deficiencies predispose hosts to septicemia from a catheter-associated UTI.Nat Commun. 2024 Mar 27;15(1):2704. doi: 10.1038/s41467-024-46974-6. Nat Commun. 2024. PMID: 38538626 Free PMC article.
Abstract
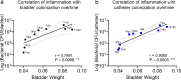
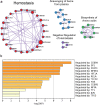
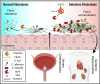
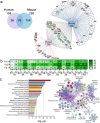
Catheter-associated urinary tract infections (CAUTIs) are amongst the most common nosocomial infections worldwide and are difficult to treat due to multi-drug resistance development among the CAUTI-related pathogens. Importantly, CAUTI often leads to secondary bloodstream infections and death. A major challenge is to predict when patients will develop CAUTIs and which populations are at-risk for bloodstream infections. Catheter-induced inflammation promotes fibrinogen (Fg) and fibrin accumulation in the bladder which are exploited as a biofilm formation platform by CAUTI pathogens. Using our established mouse model of CAUTI, we identified that host populations exhibiting either genetic or acquired fibrinolytic-deficiencies, inducing fibrin deposition in the catheterized bladder, are predisposed to severe CAUTI and septicemia by diverse uropathogens in mono- and poly-microbial infections. Furthermore, we found that E. faecalis, a prevalent CAUTI pathogen, uses the secreted protease, SprE, to induce fibrin accumulation and create a niche ideal for growth, biofilm formation, and persistence during CAUTI.
Keywords: C. albicans; CAUTI; E. coli; E. faecalis; bacterial proteases; dissemination; fibrin(ogen); fibrinolysis; inflammation; polymicrobial.
Conflict of interest statement
Declaration of interests: The authors declare no competing financial interests.
Figures







References
-
- Haider M. Z. & Annamaraju P. in StatPearls (2022).
-
- Andersen M. J. & Flores-Mireles A. L. Urinary Catheter Coating Modifications: The Race against Catheter-Associated Infections. Coatings 10, doi:ARTN 2310.3390/coatings10010023 (2020).
METHODS REFERENCES
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

