This is a preprint.
Cryo-EM structure of Alzheimer's disease tau filaments with PET ligand MK-6240
- PMID: 37790438
- PMCID: PMC10542181
- DOI: 10.1101/2023.09.22.558671
Cryo-EM structure of Alzheimer's disease tau filaments with PET ligand MK-6240
Update in
-
Cryo-EM structure of Alzheimer's disease tau filaments with PET ligand MK-6240.Nat Commun. 2024 Oct 1;15(1):8497. doi: 10.1038/s41467-024-52265-x. Nat Commun. 2024. PMID: 39353896 Free PMC article.
Abstract
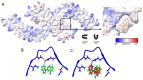
Positron Emission Tomography (PET) ligands have advanced Alzheimer's disease (AD) diagnosis and treatment. Using autoradiography and cryo-EM, we identified AD brain tissue with elevated tau burden, purified filaments, and determined the structure of second-generation high avidity PET ligand MK-6240 at 2.31 Å resolution, which bound at a 1:1 ratio within the cleft of tau paired-helical filament (PHF), engaging with glutamine 351, lysine K353, and isoleucine 360. This information elucidates the basis of MK-6240 PET in quantifying PHF deposits in AD and may facilitate the structure-based design of superior ligands against tau amyloids.
Keywords: Alzheimer’s disease (AD); Cryo-electron microscopy (cryo-EM); Neurofibrillary tangles (NFTs); Positron emission tomography (PET); Tau; [18F]MK-6240 PET tracer; atomic resolution; autoradiography; binding affinity; binding agents; in vivo PET imaging; pi-pi aromatic stacking.
Conflict of interest statement
Competing Interests The authors declare no competing interests.
Figures

References
-
- Yagishita S., Itoh Y., Nan W. & Amano N. Reappraisal of the fine structure of Alzheimer’s neurofibrillary tangles. Acta Neuropathologica 54, 239–246 (1981). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
