Wrangling Whole Mixtures Risk Assessment: Recent Advances in Determining Sufficient Similarity
- PMID: 37790747
- PMCID: PMC10545370
- DOI: 10.1016/j.cotox.2023.100417
Wrangling Whole Mixtures Risk Assessment: Recent Advances in Determining Sufficient Similarity
Abstract
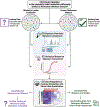
Human health risk assessments for complex mixtures can address real-world exposures and protect public health. While risk assessors typically prefer whole mixture approaches over component-based approaches, data from the precise exposure of interest are often unavailable and surrogate data from a sufficiently similar mixture(s) are required. This review describes recent advances in determining sufficient similarity of whole, complex mixtures spanning the comparison of chemical features, bioactivity profiles, and statistical evaluation to determine "thresholds of similarity". Case studies, including water disinfection byproducts, botanical ingredients, and wildfire emissions, are used to highlight tools and methods. Limitations to application of sufficient similarity in risk-based decision making are reviewed and recommendations presented for developing best practice guidelines.
Keywords: botanicals; complex mixtures; new approach methodologies; non-targeted chemical analysis; water disinfection byproducts; wildfire smoke.
Conflict of interest statement
Competing interests The authors declare that they have no competing interests. Declaration of interests The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
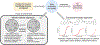

References
-
- US EPA: Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures. Edited by. Washington, DC: Risk Assessment Forum, U.S. Environmental Protection Agency; 2000.
-
- US EPA: Health assessment document for diesel engine exhaust. Edited by Assessment NCfE. Washington DC; 2002.
Grants and funding
LinkOut - more resources
Full Text Sources
