The international clinical trials registry platform (ICTRP): data integrity and the trends in clinical trials, diseases, and drugs
- PMID: 37790806
- PMCID: PMC10544909
- DOI: 10.3389/fphar.2023.1228148
The international clinical trials registry platform (ICTRP): data integrity and the trends in clinical trials, diseases, and drugs
Abstract
Introduction: Clinical trials are the gold standard for testing new therapies. Databases like ClinicalTrials.gov provide access to trial information, mainly covering the US and Europe. In 2006, WHO introduced the global ICTRP, aggregating data from ClinicalTrials.gov and 17 other national registers, making it the largest clinical trial platform by June 2019. This study conducts a comprehensive global analysis of the ICTRP database and provides framework for large-scale data analysis, data preparation, curation, and filtering. Materials and methods: The trends in 689,793 records from the ICTRP database (covering trials registered from 1990 to 2020) were analyzed. Records were adjusted for duplicates and mapping of agents to drug classes was performed. Several databases, including DrugBank, MESH, and the NIH Drug Information Portal were used to investigate trends in agent classes. Results: Our novel approach unveiled that 0.5% of the trials we identified were hidden duplicates, primarily originating from the EUCTR database, which accounted for 82.9% of these duplicates. However, the overall number of hidden duplicates within the ICTRP seems to be decreasing. In total, 689 793 trials (478 345 interventional) were registered in the ICTRP between 1990 and 2020, surpassing the count of trials in ClinicalTrials.gov (362 500 trials by the end of 2020). We identified 4 865 unique agents in trials with DrugBank, whereas 2 633 agents were identified with NIH Drug Information Portal data. After the ClinicalTrials.gov, EUCTR had the most trials in the ICTRP, followed by CTRI, IRCT, CHiCTR, and ISRCTN. CHiCTR displayed a significant surge in trial registration around 2015, while CTRI experienced rapid growth starting in 2016. Conclusion: This study highlights both the strengths and weaknesses of using the ICTRP as a data source for analyzing trends in clinical trials, and emphasizes the value of utilizing multiple registries for a comprehensive analysis.
Keywords: DrugBank; ICTRP; clinical trials; hidden duplicates; trends; trends ICTRP.
Copyright © 2023 Namiot, Smirnovová, Sokolov, Chubarev, Tarasov and Schiöth.
Conflict of interest statement
VC and VT were employed by the Limited Liable Company (LLC). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
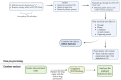

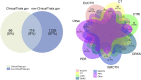




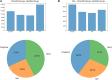
References
-
- Ali M., Ezzat A. (2020). DrugBank database XML parser. https://cran.r-project.org/web/packages/dbparser/vignettes/dbparser.html.
LinkOut - more resources
Full Text Sources

