A comprehensive overview on antibody-drug conjugates: from the conceptualization to cancer therapy
- PMID: 37790810
- PMCID: PMC10544916
- DOI: 10.3389/fphar.2023.1274088
A comprehensive overview on antibody-drug conjugates: from the conceptualization to cancer therapy
Abstract
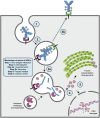
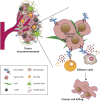
Antibody-Drug Conjugates (ADCs) represent an innovative class of potent anti-cancer compounds that are widely used in the treatment of hematologic malignancies and solid tumors. Unlike conventional chemotherapeutic drug-based therapies, that are mainly associated with modest specificity and therapeutic benefit, the three key components that form an ADC (a monoclonal antibody bound to a cytotoxic drug via a chemical linker moiety) achieve remarkable improvement in terms of targeted killing of cancer cells and, while sparing healthy tissues, a reduction in systemic side effects caused by off-tumor toxicity. Based on their beneficial mechanism of action, 15 ADCs have been approved to date by the market approval by the Food and Drug Administration (FDA), the European Medicines Agency (EMA) and/or other international governmental agencies for use in clinical oncology, and hundreds are undergoing evaluation in the preclinical and clinical phases. Here, our aim is to provide a comprehensive overview of the key features revolving around ADC therapeutic strategy including their structural and targeting properties, mechanism of action, the role of the tumor microenvironment and review the approved ADCs in clinical oncology, providing discussion regarding their toxicity profile, clinical manifestations and use in novel combination therapies. Finally, we briefly review ADCs in other pathological contexts and provide key information regarding ADC manufacturing and analytical characterization.
Keywords: antibodies; antibody-drug conjugate; cancer treatment; linkers; payloads; target therapy.
Copyright © 2023 Riccardi, Dal Bo, Macor and Toffoli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Adams G. P., Schier R., McCall A. M., Simmons H. H., Horak E. M., Alpaugh R. K., et al. (2001). High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res. 61, 4750–4755. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

