Unveiling drug-tolerant and persister-like cells in Leishmania braziliensis lines derived from patients with cutaneous leishmaniasis
- PMID: 37790908
- PMCID: PMC10543814
- DOI: 10.3389/fcimb.2023.1253033
Unveiling drug-tolerant and persister-like cells in Leishmania braziliensis lines derived from patients with cutaneous leishmaniasis
Abstract
Introduction: Resistance against anti-Leishmania drugs (DR) has been studied for years, giving important insights into long-term adaptations of these parasites to drugs, through genetic modifications. However, microorganisms can also survive lethal drug exposure by entering into temporary quiescence, a phenomenon called drug tolerance (DT), which is rather unexplored in Leishmania.
Methods: We studied a panel of nine Leishmania braziliensis strains highly susceptible to potassium antimonyl tartrate (PAT), exposed promastigotes to lethal PAT pressure, and compared several cellular and molecular parameters distinguishing DT from DR.
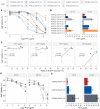
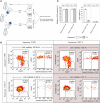
Results and discussion: We demonstrated in vitro that a variable proportion of cells remained viable, showing all the criteria of DT and not of DR: i) signatures of quiescence, under drug pressure: reduced proliferation and significant decrease of rDNA transcription; ii) reversibility of the phenotype: return to low IC50 after removal of drug pressure; and iii) absence of significant genetic differences between exposed and unexposed lineages of each strain and absence of reported markers of DR. We found different levels of quiescence and DT among the different L. braziliensis strains. We provide here a new in-vitro model of drug-induced quiescence and DT in Leishmania. Research should be extended in vivo, but the current model could be further exploited to support R&D, for instance, to guide the screening of compounds to overcome the quiescence resilience of the parasite, thereby improving the therapy of leishmaniasis.
Keywords: Leishmania; antimonials; drug tolerance; persisters; quiescence.
Copyright © 2023 Jara, Arevalo, Llanos-Cuentas, Broeck, Domagalska and Dujardin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Armitage E. G., Alqaisi A. Q. I., Godzien J., Peña I., Mbekeani A. J., Alonso-Herranz V., et al. (2018). Complex interplay between sphingolipid and sterol metabolism revealed by perturbations to the leishmania metabolome caused by miltefosine. Antimicrob. Agents Chemother. 62, e02095-17. doi: 10.1128/AAC.02095-17 - DOI - PMC - PubMed
-
- Basu J. M., Mookerjee A., Sen P., Bhaumik S., Sen P., Banerjee S., et al. (2006). Sodium antimony gluconate induces generation of reactive oxygen species and nitric oxide via phosphoinositide 3-kinase and mitogen-activated protein kinase activation in Leishmania donovani-infected macrophages. Antimicrob. Agents Chemother. 50, 1788–1797. doi: 10.1128/AAC.50.5.1788-1797.2006 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

