Comparative Study of Guanidine-, Acetamidine- and Urea-Based Chloroaluminate Electrolytes for an Aluminum Battery
- PMID: 37791096
- PMCID: PMC10544989
- DOI: 10.1021/acs.jpcc.3c05287
Comparative Study of Guanidine-, Acetamidine- and Urea-Based Chloroaluminate Electrolytes for an Aluminum Battery
Abstract
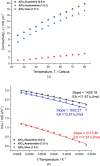
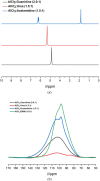
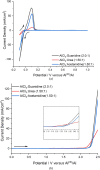
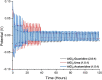
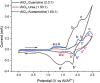
Aluminum-based batteries are a promising alternative to lithium-ion as they are considered to be low-cost and more friendly to the environment. In addition, aluminum is abundant and evenly distributed across the globe. Many studies and Al battery prototypes use imidazolium chloroaluminate electrolytes because of their good rheological and electrochemical performance. However, these electrolytes are very expensive, and so cost is a barrier to industrial scale-up. A urea-based electrolyte, AlCl3:Urea, has been proposed as an alternative, but its performance is relatively poor because of its high viscosity and low conductivity. This type of electrolyte has become known as an ionic liquid analogue (ILA). In this contribution, we proposed two Lewis base salt precursors, namely, guanidine hydrochloride and acetamidine hydrochloride, as alternatives to the urea-based ILA. We present the study of three ILAs, AlCl3:Guanidine, AlCl3:Acetamidine, and AlCl3:Urea, examining their rheology, electrochemistry, NMR spectra, and coin-cell performance. The room temperature viscosities of both AlCl3:Guanidine (52.9 cP) and AlCl3:Acetamidine (76.0 cP) were significantly lower than those of the urea-based liquid (240.9 cP), and their conductivities were correspondingly higher. Cyclic voltammetry (CV) and linear sweep voltammetry (LSV) showed that all three electrolytes exhibit reversible deposition/dissolution of Al, but LSV indicated that AlCl3:Guanidine and AlCl3:Acetamidine ILAs have superior anodic stability compared to the AlCl3:Urea electrolyte, as evidenced by anodic potential limits of +2.23 V for both AlCl3:Guanidine and AlCl3:Acetamidine and +2.12 V for AlCl3:Urea. Coin-cell tests showed that both AlCl3:Guanidine and AlCl3:Acetamidine ILA exhibit a higher Coulombic efficiency (98 and 97%, respectively) than the AlCl3:Urea electrolyte system, which has an efficiency of 88% after 100 cycles at 60 mA g-1. Overall, we show that AlCl3:Guanidine and AlCl3:Acetamidine have superior performance when compared to AlCl3:Urea, while maintaining low economic cost. We consider these to be valuable alternative materials for Al-based battery systems, especially for commercial production.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Agiorgousis M. L.; Sun Y.-Y.; Zhang S. The Role of Ionic Liquid Electrolyte in an Aluminum–Graphite Electrochemical Cell. ACS Energy Lett. 2017, 2 (3), 689–693. 10.1021/acsenergylett.7b00110. - DOI
-
- Elia G. A.; Kravchyk K. V.; Kovalenko M. V.; Chacón J.; Holland A.; Wills R. G. A. An overview and prospective on Al and Al-ion battery technologies. J. Power Sources 2021, 481, 22887010.1016/j.jpowsour.2020.228870. - DOI
-
- Han X.; Bai Y.; Zhao R.; Li Y.; Wu F.; Wu C. Electrolytes for rechargeable aluminum batteries. Prog. Mater. Sci. 2022, 128, 10096010.1016/j.pmatsci.2022.100960. - DOI
-
- Song Y.; Jiao S.; Tu J.; Wang J.; Liu Y.; Jiao H.; et al. A long-life rechargeable Al ion battery based on molten salts. J. Mater. Chem. A 2017, 5 (3), 1282–1291. 10.1039/C6TA09829K. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
