C5aR1 blockade reshapes immunosuppressive tumor microenvironment and synergizes with immune checkpoint blockade therapy in high-grade serous ovarian cancer
- PMID: 37791232
- PMCID: PMC10543342
- DOI: 10.1080/2162402X.2023.2261242
C5aR1 blockade reshapes immunosuppressive tumor microenvironment and synergizes with immune checkpoint blockade therapy in high-grade serous ovarian cancer
Abstract
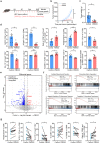
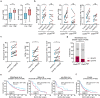
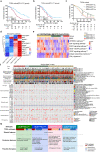
High-grade serous ovarian cancer (HGSC), with a modest response to immune checkpoint blockade (ICB) targeting PD-1/PD-L1 monotherapy, is densely infiltrated by M2-polarized tumor-associated macrophages (TAMs) and regulatory T (Treg) cells. The complement C5a/C5aR1 axis contributes to the programming of the immunosuppressive phenotype of TAMs in solid tumors and represents a promising immunomodulatory target for treating HGSCs. Here, we aimed to identify the relevance of C5aR1 in prognosis, immune microenvironment, and immunotherapy response in HGSCs. The expression and relationship of C5aR1 with tumor-infiltrating immune cells were assessed by immunohistochemistry and flow cytometry in the training cohort (n = 120) and fresh HGSC tissues (n = 36). Transcriptomic analyses of the xenografts delineated the mechanisms driving the immunomodulatory activity of PMX53, an orally bioavailable C5aR1 inhibitor. Therapeutic relevance was confirmed in ex vivo tumor cultures and The Cancer Genome Atlas (TCGA) datasets. C5aR1 expression independently predicted dismal prognosis and was linked to the immunoevasive subtype of HGSC, characterized by increased infiltration of pro-tumor cells (Treg cells, M2-polarized macrophages, and neutrophils) and impaired CD8+T functions. PMX53 antagonized subcutaneous tumor growth, modulated immunosuppressive mechanisms and synergized with aPD-1 in several tumor types. Single-cell RNA-seq analysis revealed predominant C5aR1 expression in TAMs, with an immunosuppressive-related expression signature in C5aR1+TAMs. Furthermore, the combination of C5aR1 and PD-L1 was associated with specific molecular characteristics and matched clinical response annotations. Therefore, the abundance of C5aR1 could predict an inferior prognosis in HGSCs, and incorporating PD-L1 may serve as a novel predictive biomarker to guide therapeutic options.
Keywords: High-grade serous ovarian cancer; immunotherapy; prognosis; tumor microenvironment; tumor-associated macrophages.
© 2023 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Disis ML, Taylor MH, Kelly K, Beck JT, Gordon M, Moore KM, Patel MR, Chaves J, Park H, Mita AC, et al. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol. 2019;5(3):393–14. doi: 10.1001/jamaoncol.2018.6258. - DOI - PMC - PubMed
-
- Matulonis UA, Shapira-Frommer R, Santin AD, Lisyanskaya AS, Pignata S, Vergote I, Raspagliesi F, Sonke GS, Birrer M, Provencher DM, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol. 2019;30(7):1080–1087. doi: 10.1093/annonc/mdz135. - DOI - PubMed
-
- Anadon CM, Yu X, Hänggi K, Biswas S, Chaurio RA, Martin A, Payne KK, Mandal G, Innamarato P, Harro CM, et al. Ovarian cancer immunogenicity is governed by a narrow subset of progenitor tissue-resident memory T cells. Cancer Cell. 2022;40(5):545–557. doi: 10.1016/j.ccell.2022.03.008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
