Empagliflozin Suppresses the Differentiation/Maturation of Human Epicardial Preadipocytes and Improves Paracrine Secretome Profile
- PMID: 37791312
- PMCID: PMC10544075
- DOI: 10.1016/j.jacbts.2023.05.007
Empagliflozin Suppresses the Differentiation/Maturation of Human Epicardial Preadipocytes and Improves Paracrine Secretome Profile
Abstract
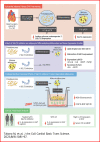
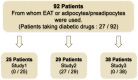
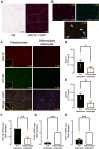
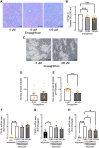
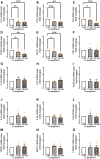
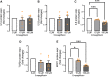
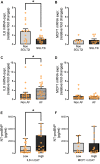
Sodium-glucose cotransporter 2 (SGLT2) inhibitors reduce epicardial adipose tissue (EAT) in humans, enhancing cardioprotective effects on heart failure and atrial fibrillation. We investigated the direct effect of the SGLT2 inhibitor empagliflozin on human primary epicardial adipocytes and preadipocytes. SGLT2 is primarily expressed in human preadipocytes in the EAT. The expression levels of SGLT2 significantly diminished when the preadipocytes were terminally differentiated. Adipogenesis of preadipocytes was attenuated by empagliflozin treatment without affecting cell proliferation. The messenger RNA levels and secreted protein levels of interleukin 6 and monocyte chemoattractant protein 1 were significantly decreased in empagliflozin-treated adipocytes. Coculture of human induced pluripotent stem cell-derived atrial cardiomyocytes and adipocytes pretreated with or without empagliflozin revealed that empagliflozin significantly suppressed reactive oxygen species. IL6 messenger RNA expression in human EAT showed significant clinically relevant associations. Empagliflozin suppresses human epicardial preadipocyte differentiation/maturation, likely inhibiting epicardial adipogenesis and improving the paracrine secretome profile of EAT, particularly by regulating IL6 expression.
Keywords: epicardial adipose tissue; interleukin 6; paracrine effect; preadipocyte; sodium-glucose cotransporter 2 inhibitor.
© 2023 Published by Elsevier on behalf of the American College of Cardiology Foundation.
Conflict of interest statement
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

References
-
- Zinman B., Wanner C., Lachin J.M., et al. EMPA-REG OUTCOME Investigators Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. - PubMed
-
- Neal B., Perkovic V., Mahaffey K.W., et al. CANVAS Program Collaborative Group Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. - PubMed
-
- Wiviott S.D., Raz I., Bonaca M.P., et al. DECLARE–TIMI 58 Investigators Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. - PubMed
-
- McMurray J.J.V., Solomon S.D., Inzucchi S.E., et al. DAPA-HF Trial Committees and Investigators Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. - PubMed
-
- Packer M., Anker S.D., Butler J., et al. EMPEROR-Reduced Trial Investigators Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

