Acute and Sub-chronic Anticonvulsant Effects of Edaravone on Seizure Induced by Pentylenetetrazole or Electroshock in Mice, Nitric Oxide Involvement
- PMID: 37791336
- PMCID: PMC10542921
- DOI: 10.30476/ijms.2022.94177.2544
Acute and Sub-chronic Anticonvulsant Effects of Edaravone on Seizure Induced by Pentylenetetrazole or Electroshock in Mice, Nitric Oxide Involvement
Abstract
Background: Edaravone is an anti-stroke medication that may have nitric oxide (NO) modulating properties. This study evaluated the role of NO in the acute and sub-chronic anticonvulsant effects of edaravone in murine models of seizures induced by intraperitoneal (IP) or intravenous (IV) injections of pentylenetetrazole (PTZ) or electroshock (maximal electroshock seizure [MES]).
Methods: 132 male albino mice were randomly divided into 22 groups (n=6) and given IP injections of vehicle or edaravone either acutely or for eight days (sub-chronically). The seizure was induced by electroshock or PTZ (IP or IV). The following edaravone doses were used: 7.5, 10, 12.5 (acute); 5, 7.5, 10 (sub-chronic) in IP PTZ model; 5, 7.5, 10 in IV PTZ model; and 5, 10 mg/Kg in the MES. To evaluate NO involvement, 216 mice were randomly divided into 36 groups (n=6) and pretreated with vehicle, edaravone, a non-specific nitric oxide synthase (NOS) inhibitor: N(ω)-nitro-L-arginine methyl ester (L-NAME) (5 mg/Kg), a specific nNOS inhibitor: 7-nitroindazole (7-NI) (60 mg/Kg), or a combination of edaravone plus L-NAME or 7-NI, either acutely or for eight days before seizure induction. Doses of edaravone were as follows: in IP PTZ model: 12.5 (acute) and 10 (sub-chronic); in IV PTZ model: 10; and in the MES: 5 mg/Kg. Data were analyzed using the one-way analysis of variance (ANOVA) followed by Tukey's test (SPSS 18). P≤0.05 was considered statistically significant.
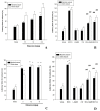
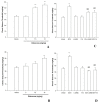
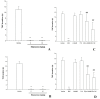
Results: In the IP PTZ model, edaravone increased time latencies to seizures (P<0.001), prevented tonic seizures, and death. Edaravone increased the seizure threshold (P<0.001) in the IV PTZ model and shortened the duration of tonic hind-limb extension (THE) in the MES model (P<0.001). In comparison to mice treated with edaravone alone, adding L-NAME or 7-NI reduced seizure time latencies (P<0.001), reduced seizure threshold (P<0.001), and increased THE duration (P<0.001).
Conclusion: Edaravone (acute or sub-chronic) could prevent seizures by modulating NO signaling pathways.
Keywords: Edaravone; Electroshock; Epilepsy; Nitric oxide; Pentylenetetrazole.
Copyright: © Iranian Journal of Medical Sciences.
Conflict of interest statement
None declared.
Figures
Similar articles
-
The possible role of nitric oxide signaling and NMDA receptors in allopurinol effect on maximal electroshock- and pentylenetetrazol-induced seizures in mice.Neurosci Lett. 2022 May 1;778:136620. doi: 10.1016/j.neulet.2022.136620. Epub 2022 Apr 5. Neurosci Lett. 2022. PMID: 35395326
-
The effect of acute aripiprazole treatment on chemically and electrically induced seizures in mice: The role of nitric oxide.Epilepsy Behav. 2015 Jul;48:35-40. doi: 10.1016/j.yebeh.2015.05.018. Epub 2015 May 31. Epilepsy Behav. 2015. PMID: 26037847
-
The effects of coenzyme Q10 on seizures in mice: the involvement of nitric oxide.Epilepsy Behav. 2014 Aug;37:36-42. doi: 10.1016/j.yebeh.2014.05.024. Epub 2014 Jun 24. Epilepsy Behav. 2014. PMID: 24972157
-
Anticonvulsant effects of pentoxifylline on seizures induced by pentylenetetrazole and maximal electroshock in male mice: The role of the nitrergic pathway.IBRO Neurosci Rep. 2024 Nov 26;17:485-492. doi: 10.1016/j.ibneur.2024.11.013. eCollection 2024 Dec. IBRO Neurosci Rep. 2024. PMID: 39717871 Free PMC article.
-
Factors influencing the acute pentylenetetrazole-induced seizure paradigm and a literature review.Ann Clin Transl Neurol. 2021 Jul;8(7):1388-1397. doi: 10.1002/acn3.51375. Epub 2021 Jun 8. Ann Clin Transl Neurol. 2021. PMID: 34102033 Free PMC article. Review.
References
-
- Lee SK. Old versus New: Why Do We Need New Antiepileptic Drugs? J Epilepsy Res. 2014;4:39–44. doi: 10.14581/jer.14010. [ PMC Free Article ] - DOI - PMC - PubMed
-
- Fattorusso A, Matricardi S, Mencaroni E, Dell’Isola GB, Di Cara G, Striano P, et al. The Pharmacoresistant Epilepsy: An Overview on Existant and New Emerging Therapies. Front Neurol. 2021;12:674483. doi: 10.3389/fneur.2021.674483. [ PMC Free Article ] - DOI - PMC - PubMed
-
- Perucca E. The pharmacological treatment of epilepsy: recent advances and future perspectives. Acta Epileptologica. 2021;3:1–11. doi: 10.1186/s42494-021-00055-z. - DOI
-
- Loscher W, Potschka H, Sisodiya SM, Vezzani A. Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options. Pharmacol Rev. 2020;72:606–38. doi: 10.1124/pr.120.019539. [ PMC Free Article ] - DOI - PMC - PubMed
-
- Watanabe K, Tanaka M, Yuki S, Hirai M, Yamamoto Y. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J Clin Biochem Nutr. 2018;62:20–38. doi: 10.3164/jcbn.17-62. [ PMC Free Article ] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
