Human pluripotent stem cell-derived inner ear organoids recapitulate otic development in vitro
- PMID: 37791525
- PMCID: PMC10565253
- DOI: 10.1242/dev.201865
Human pluripotent stem cell-derived inner ear organoids recapitulate otic development in vitro
Abstract
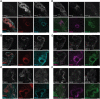
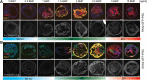
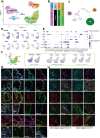
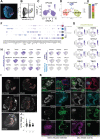
Our molecular understanding of the early stages of human inner ear development has been limited by the difficulty in accessing fetal samples at early gestational stages. As an alternative, previous studies have shown that inner ear morphogenesis can be partially recapitulated using induced pluripotent stem cells directed to differentiate into inner ear organoids (IEOs). Once validated and benchmarked, these systems could represent unique tools to complement and refine our understanding of human otic differentiation and model developmental defects. Here, we provide the first direct comparisons of the early human embryonic otocyst and fetal sensory organs with human IEOs. We use multiplexed immunostaining and single-cell RNA-sequencing to characterize IEOs at three key developmental steps, providing a new and unique signature of in vitro-derived otic placode, epithelium, neuroblasts and sensory epithelia. In parallel, we evaluate the expression and localization of crucial markers at these equivalent stages in human embryos. Together, our data indicate that the current state-of-the-art protocol enables the specification of bona fide otic tissue, supporting the further application of IEOs to inform inner ear biology and disease.
Keywords: Hair cells; Human iPSC-derived inner ear organoids; Human otocyst; Inner ear development; Otic neuroblasts; Sensory epithelia.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests K.R.K. is an inventor on patents related to the inner ear organoid system and consults for STEMCELL Technologies on matters related to the technology. The other authors declare no competing interests.
Figures


Update of
-
Human pluripotent stem cells-derived inner ear organoids recapitulate otic development in vitro.bioRxiv [Preprint]. 2023 Apr 12:2023.04.11.536448. doi: 10.1101/2023.04.11.536448. bioRxiv. 2023. Update in: Development. 2023 Oct 1;150(19):dev201865. doi: 10.1242/dev.201865. PMID: 37090562 Free PMC article. Updated. Preprint.
References
-
- Adameyko, I., Lallemend, F., Furlan, A., Zinin, N., Aranda, S., Kitambi, S. S., Blanchart, A., Favaro, R., Nicolis, S., Lubke, M.et al. (2012). Sox2 and Mitf cross-regulatory interactions consolidate progenitor and melanocyte lineages in the cranial neural crest. Development 139, 397-410. 10.1242/dev.065581 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

